Diversifying molecular and topological space via a supramolecular solid-state synthesis: a purely organic mok net sustained by hydrogen bonds
- PMID: 31709059
- PMCID: PMC6830215
- DOI: 10.1107/S2052252519011382
Diversifying molecular and topological space via a supramolecular solid-state synthesis: a purely organic mok net sustained by hydrogen bonds
Abstract
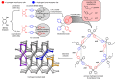
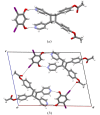
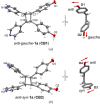
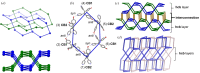
A three-dimensional hydrogen-bonded network based on a rare mok topology has been constructed using an organic molecule synthesized in the solid state. The molecule is obtained using a supramolecular protecting-group strategy that is applied to a solid-state [2+2] photodimerization. The photodimerization affords a novel head-to-head cyclo-butane product. The cyclo-butane possesses tetrahedrally disposed cis-hydrogen-bond donor (phenolic) and cis-hydrogen-bond acceptor (pyridyl) groups. The product self-assembles in the solid state to form a mok network that exhibits twofold interpenetration. The cyclo-butane adopts different conformations to provide combinations of hydrogen-bond donor and acceptor sites to conform to the structural requirements of the mok net.
Keywords: [2+2] photocycloaddition; co-crystals; crystal engineering; intermolecular interactions; organic solid-state reactions; supramolecular chemistry; three-dimensional hydrogen-bonded organic networks.
© Shalisa M. Oburn et al. 2019.
Figures


Similar articles
-
Halogen-bonded co-crystal containing 1,3-di-iodo-perchloro-benzene and the photoproduct rtct-tetra-kis-(pyridin-4-yl)cyclo-butane resulting in a zigzag topology.Acta Crystallogr E Crystallogr Commun. 2023 Feb 21;79(Pt 3):212-215. doi: 10.1107/S2056989023001408. eCollection 2023 Feb 1. Acta Crystallogr E Crystallogr Commun. 2023. PMID: 36910002 Free PMC article.
-
Supramolecular control of reactivity in the solid state: from templates to ladderanes to metal-organic frameworks.Acc Chem Res. 2008 Feb;41(2):280-91. doi: 10.1021/ar700145r. Epub 2008 Feb 19. Acc Chem Res. 2008. PMID: 18281948
-
Structural flexibility of halogen bonds showed in a single-crystal-to-single-crystal [2+2] photodimerization.IUCrJ. 2018 Jun 22;5(Pt 4):491-496. doi: 10.1107/S2052252518007583. eCollection 2018 Jul 1. IUCrJ. 2018. PMID: 30002849 Free PMC article.
-
Supramolecular Matter Through Crystal Engineering: Covalent Bond Formation to Postsynthetic Modification.Chemistry. 2025 May 14;31(27):e202500756. doi: 10.1002/chem.202500756. Epub 2025 Apr 21. Chemistry. 2025. PMID: 40193221 Review.
-
Urea Derivatives as Functional Molecules: Supramolecular Capsules, Supramolecular Polymers, Supramolecular Gels, Artificial Hosts, and Catalysts.Chemistry. 2021 Mar 26;27(18):5601-5614. doi: 10.1002/chem.202004367. Epub 2021 Jan 29. Chemistry. 2021. PMID: 33184975 Review.
Cited by
-
Crystal engineering in all its hues in IUCrJ.IUCrJ. 2020 Feb 29;7(Pt 2):143. doi: 10.1107/S2052252520001608. eCollection 2020 Mar 1. IUCrJ. 2020. PMID: 32148840 Free PMC article.
-
Halogen-Bond Mediated [2+2] Photodimerizations: À la Carte Access to Unsymmetrical Cyclobutanes in the Solid State.Molecules. 2022 Feb 3;27(3):1048. doi: 10.3390/molecules27031048. Molecules. 2022. PMID: 35164313 Free PMC article.
References
-
- Alexandrov, E. V., Blatov, V. A., Kochetkov, A. V. & Proserpio, D. M. (2011). CrystEngComm, 13, 3947–3958.
-
- Alexandrov, E. V., Blatov, V. A. & Proserpio, D. M. (2012). Acta Cryst. A68, 484–493.
-
- Baburin, I. A., Blatov, V. A., Carlucci, L., Ciani, G. & Proserpio, D. M. (2008). Cryst. Growth Des. 8, 519–539.
-
- Biradha, K. & Santra, R. (2013). Chem. Soc. Rev. 42, 950–967. - PubMed
-
- Blake, A. J., Champness, N. R., Chung, S. S. M., Li, W.-S. & Schröder, M. (1997). Chem. Commun. pp. 1675–1676.
LinkOut - more resources
Full Text Sources

