Diversifying molecular and topological space via a supramolecular solid-state synthesis: a purely organic mok net sustained by hydrogen bonds
- PMID: 31709059
- PMCID: PMC6830215
- DOI: 10.1107/S2052252519011382
Diversifying molecular and topological space via a supramolecular solid-state synthesis: a purely organic mok net sustained by hydrogen bonds
Abstract
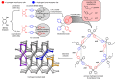
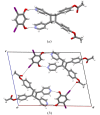
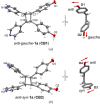
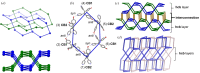
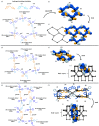
A three-dimensional hydrogen-bonded network based on a rare mok topology has been constructed using an organic molecule synthesized in the solid state. The molecule is obtained using a supramolecular protecting-group strategy that is applied to a solid-state [2+2] photodimerization. The photodimerization affords a novel head-to-head cyclo-butane product. The cyclo-butane possesses tetrahedrally disposed cis-hydrogen-bond donor (phenolic) and cis-hydrogen-bond acceptor (pyridyl) groups. The product self-assembles in the solid state to form a mok network that exhibits twofold interpenetration. The cyclo-butane adopts different conformations to provide combinations of hydrogen-bond donor and acceptor sites to conform to the structural requirements of the mok net.
Keywords: [2+2] photocycloaddition; co-crystals; crystal engineering; intermolecular interactions; organic solid-state reactions; supramolecular chemistry; three-dimensional hydrogen-bonded organic networks.
© Shalisa M. Oburn et al. 2019.
Figures


References
-
- Alexandrov, E. V., Blatov, V. A., Kochetkov, A. V. & Proserpio, D. M. (2011). CrystEngComm, 13, 3947–3958.
-
- Alexandrov, E. V., Blatov, V. A. & Proserpio, D. M. (2012). Acta Cryst. A68, 484–493.
-
- Baburin, I. A., Blatov, V. A., Carlucci, L., Ciani, G. & Proserpio, D. M. (2008). Cryst. Growth Des. 8, 519–539.
-
- Biradha, K. & Santra, R. (2013). Chem. Soc. Rev. 42, 950–967. - PubMed
-
- Blake, A. J., Champness, N. R., Chung, S. S. M., Li, W.-S. & Schröder, M. (1997). Chem. Commun. pp. 1675–1676.
LinkOut - more resources
Full Text Sources

