Consistency and variability of cocrystals containing positional isomers: the self-assembly evolution mechanism of supramolecular synthons of cresol-piperazine
- PMID: 31709062
- PMCID: PMC6830220
- DOI: 10.1107/S2052252519012363
Consistency and variability of cocrystals containing positional isomers: the self-assembly evolution mechanism of supramolecular synthons of cresol-piperazine
Abstract
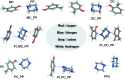
The disposition of functional groups can induce variations in the nature and type of interactions and hence affect the molecular recognition and self-assembly mechanism in cocrystals. To better understand the formation of cocrystals on a molecular level, the effects of disposition of functional groups on the formation of cocrystals were systematically and comprehensively investigated using cresol isomers (o-, m-, p-cresol) as model compounds. Consistency and variability in these cocrystals containing positional isomers were found and analyzed. The structures, molecular recognition and self-assembly mechanism of supramolecular synthons in solution and in their corresponding cocrystals were verified by a combined experimental and theoretical calculation approach. It was found that the heterosynthons (heterotrimer or heterodimer) combined with O-H⋯N hydrogen bonding played a significant role. Hirshfeld surface analysis and computed interaction energy values were used to determine the hierarchical ordering of the weak interactions. The quantitative analyses of charge transfers and molecular electrostatic potential were also applied to reveal and verify the reasons for consistency and variability. Finally, the molecular recognition, self-assembly and evolution process of the supramolecular synthons in solution were investigated. The results confirm that the supramolecular synthon structures formed initially in solution would be carried over to the final cocrystals, and the supramolecular synthon structures are the precursors of cocrystals and the information memory of the cocrystallization process, which is evidence for classical nucleation theory.
Keywords: co-crystals; cocrystal consistency; cocrystal variability; density functional theory; hydrogen bonding; intermolecular interactions; lattice energy; quantum chemistry; supramolecular synthons; theoretical calculations.
© Wang et al. 2019.
Figures





References
-
- Alecu, I. M., Zheng, J., Zhao, Y. & Truhlar, D. G. (2010). J. Chem. Theory Comput. 6, 2872–2887. - PubMed
-
- Almarsson, Ö. & Zaworotko, M. J. (2004). Chem. Commun. 0, 1889–1896. - PubMed
-
- Antony, J., Sure, R. & Grimme, S. (2015). Chem. Commun. 51, 1764–1774. - PubMed
-
- Arunanl, E., Desiraju, G. R., Klein, R. A., Sadlej, J., Scheiner, S., Alkorta, I., Clary, D. C., Crabtree, R. H., Dannenberg, J. J., Hobza, P., Kjaergaard, H. G., Legon, A. C., Mennucci, B. & Nesbitt, D. J. (2011). Pure Appl. Chem. 83, 1619–1636.
-
- Bahers, T. Le, Adamo, C. & Ciofini, I. (2011). J. Chem. Theory Comput. 7, 2498–2506. - PubMed
LinkOut - more resources
Full Text Sources

