One-dimensional ladder gallium coordination polymer
- PMID: 31709077
- PMCID: PMC6829714
- DOI: 10.1107/S2056989019013446
One-dimensional ladder gallium coordination polymer
Abstract
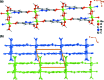
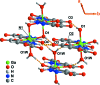
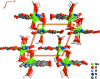
A one-dimensional ladder-type coordination polymer, poly[[(μ2-hydroxido)(μ2-1H-pyrazole-3,5-di-carboxyl-ato)gallium(III)] monohydrate], [Ga(C5H2N2O4)(OH)(H2O)] n or [Ga(HPDC)(OH)(H2O)] n , I, isotypic with a V3+ coordination polymer previously reported by Chen et al. [J. Coord. Chem. (2008). 61, 3556-3567] was prepared from Ga3+ and pyrazole-3,5-di-carb-oxy-lic acid monohydrate (H3PDC·H2O). Compound I was isolated using three distinct experimental methods: hydro-thermal (HT), microwave-assisted (MWAS) and one-pot (OP) and the crystallite size should be fine-tuned according to the method employed. The coordination polymeric structure is based on a dimeric Ga3+ moiety comprising two μ2-bridging hydroxide groups, which are inter-connected by HPDC2- anionic organic linkers. The close packing of individual polymers is strongly directed by the supra-molecular inter-actions, namely several O-H⋯O and N-H⋯O hydrogen-bonding inter-actions.
Keywords: coordination polymers; crystal structure; gallium.
© Simões et al. 2019.
Figures


References
-
- Ajoyan, Z., Marino, P. & Howarth, A. J. (2018). CrystEngComm, 20, 5899–5912.
-
- Banerjee, D., Kim, S. J., Wu, H., Xu, W., Borkowski, L. A., Li, J. & Parise, J. B. (2011). Inorg. Chem. 50, 208–212. - PubMed
-
- Brandenburg, K. (1999). DIAMOND. Crystal Impact GbR, Bonn, Germany.
-
- Bruker (2001). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2015). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
