Crystal structure, Hirshfeld surface analysis and DFT studies of ethyl 2-{4-[(2-eth-oxy-2-oxoeth-yl)(phen-yl)carbamo-yl]-2-oxo-1,2-di-hydro-quinolin-1-yl}acetate
- PMID: 31709103
- PMCID: PMC6829727
- DOI: 10.1107/S2056989019014154
Crystal structure, Hirshfeld surface analysis and DFT studies of ethyl 2-{4-[(2-eth-oxy-2-oxoeth-yl)(phen-yl)carbamo-yl]-2-oxo-1,2-di-hydro-quinolin-1-yl}acetate
Abstract
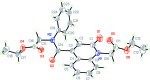
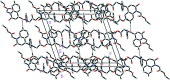
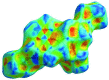
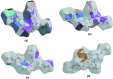
The title com-pound, C24H24N2O6, consists of ethyl 2-(1,2,3,4-tetra-hydro-2-oxo-quinolin-1-yl)acetate and 4-[(2-eth-oxy-2-oxoeth-yl)(phen-yl)carbomoyl] units, where the oxo-quinoline unit is almost planar and the acetate substituent is nearly perpendicular to its mean plane. In the crystal, C-HOxqn⋯OEthx and C-HPh-yl⋯OCarbx (Oxqn = oxoquinolin, Ethx = eth-oxy, Phyl = phenyl and Carbx = carboxyl-ate) weak hydrogen bonds link the mol-ecules into a three-dimensional network sturucture. A π-π inter-action between the constituent rings of the oxo-quinoline unit, with a centroid-centroid distance of 3.675 (1) Å may further stabilize the structure. Both terminal ethyl groups are disordered over two sets of sites. The ratios of the refined occupanies are 0.821 (8):0.179 (8) and 0.651 (18):0.349 (18). The Hirshfeld surface analysis of the crystal structure indicates that the most important contributions for the crystal packing are from H⋯H (53.9%), H⋯O/O⋯H (28.5%) and H⋯C/C⋯H (11.8%) inter-actions. Weak inter-molecular hydrogen-bond inter-actions and van der Waals inter-actions are the dominant inter-actions in the crystal packing. Density functional theory (DFT) geometric optimized structures at the B3LYP/6-311G(d,p) level are com-pared with the experimentally determined mol-ecular structure in the solid state. The HOMO-LUMO mol-ecular orbital behaviour was elucidated to determine the energy gap.
Keywords: 2-oxoquinoline; Hirshfeld surface; alkyne; crystal structure; weak intermolecular interactions; π-stacking.
© Filali Baba et al. 2019.
Figures




Similar articles
-
Crystal structure, Hirshfeld surface analysis and inter-action energy and DFT studies of 2-chloro-ethyl 2-oxo-1-(prop-2-yn-1-yl)-1,2-di-hydro-quinoline-4-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2019 Sep 6;75(Pt 10):1411-1417. doi: 10.1107/S2056989019012283. eCollection 2019 Oct 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31636967 Free PMC article.
-
Crystal structure, Hirshfeld surface analysis and inter-action energy and DFT studies of 3-{(2Z)-2-[(2,4-di-chloro-phen-yl)methyl-idene]-3-oxo-3,4-di-hydro-2H-1,4-benzo-thia-zin-4-yl}propane-nitrile.Acta Crystallogr E Crystallogr Commun. 2019 May 3;75(Pt 6):721-727. doi: 10.1107/S2056989019005966. eCollection 2019 Jun 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31391953 Free PMC article.
-
Crystal structure, Hirshfeld surface analysis and DFT studies of 5-bromo-1-{2-[2-(2-chloro-eth-oxy)eth-oxy]eth-yl}indoline-2,3-dione.Acta Crystallogr E Crystallogr Commun. 2019 Aug 30;75(Pt 9):1372-1378. doi: 10.1107/S2056989019011617. eCollection 2019 Sep 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31523469 Free PMC article.
-
Crystal structure, Hirshfeld surface analysis and inter-action energy and DFT studies of methyl 4-[3,6-bis-(pyridin-2-yl)pyridazin-4-yl]benzoate.Acta Crystallogr E Crystallogr Commun. 2019 Oct 22;75(Pt 11):1672-1678. doi: 10.1107/S2056989019013732. eCollection 2019 Nov 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31709088 Free PMC article.
-
Crystal structure, Hirshfeld surface analysis and DFT study of (2Z)-2-(2,4-di-chloro-benzyl-idene)-4-[2-(2-oxo-1,3-oxazolidin-3-yl)eth-yl]-3,4-di-hydro-2H-1,4-benzo-thia-zin-3-one.Acta Crystallogr E Crystallogr Commun. 2019 Apr 9;75(Pt 5):593-599. doi: 10.1107/S2056989019004250. eCollection 2019 May 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31110793 Free PMC article.
References
-
- Ahmed, N., Brahmbhatt, K. G., Sabde, S., Mitra, D., Singh, I. P. & Bhutani, K. K. (2010). Bioorg. Med. Chem. 18, 2872–2879. - PubMed
-
- Becke, A. D. (1993). J. Chem. Phys. 98, 5648–5652.
-
- Bouzian, Y., Hlimi, F., Sebbar, N. K., El Hafi, M., Hni, B., Essassi, E. M. & Mague, J. T. (2018). IUCrData, 3, x181438.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
