Production of Melanins With Recombinant Microorganisms
- PMID: 31709247
- PMCID: PMC6821874
- DOI: 10.3389/fbioe.2019.00285
Production of Melanins With Recombinant Microorganisms
Abstract
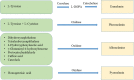
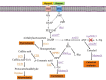
The melanins constitute a diverse group of natural products found in most organisms, having functions related to protection against chemical and physical stresses. These products originate from the enzyme-catalyzed oxidation of phenolic and indolic substrates that polymerize to yield melanins, which include eumelanin, pheomelanin, pyomelanin, and the allomelanins. The enzymes involved in melanin formation belong mainly to the tyrosinase and laccase protein families. The melanins are polymeric materials having applications in the pharmaceutical, cosmetic, optical, and electronic industries. The biotechnological production of these polymers is an attractive alternative to obtaining them by extraction from plant or animal material, where they are present at low concentrations. Several species of microorganisms have been identified as having a natural melanogenic capacity. The development and optimization of culture conditions with these organisms has resulted in processes for generating melanins. These processes are based on the conversion of melanin precursors present in the culture medium to the corresponding polymers. With the application of genetic engineering techniques, it has become possible to overexpress genes encoding enzymes involved in melanin formation, mostly tyrosinases, leading to an improvement in the productivity of melanogenic organisms, as well as allowing the generation of novel recombinant microbial strains that can produce diverse types of melanins. Furthermore, the metabolic engineering of microbial hosts by modifying pathways related to the supply of melanogenic precursors has resulted in strains with the capacity of performing the total synthesis of melanins from simple carbon sources in the scale of grams. In this review, the latest advances toward the generation of recombinant melanin production strains and production processes are summarized and discussed.
Keywords: aromatics; melanin; metabolic engineering; process engineering; tyrosinase.
Copyright © 2019 Martínez, Martinez and Gosset.
Figures

References
-
- Ahn S. Y., Choi M., Jeong D. W., Park S., Park H., Jang K. S., et al. (2019). Synthesis and chemical composition analysis of protocatechualdehyde-based novel melanin dye by 15T FT-ICR: high dyeing performance on soft contact lens. Dyes and Pigments 160, 546–554. 10.1016/j.dyepig.2018.08.058 - DOI
-
- Ambrico M., Vecchia N. F. D., Ambrico P. F., Cardone A., Cicco S. R., Ligonzo T., et al. (2014). A photoresponsive red-hair-inspired polydopamine-based copolymer for hybrid photocapacitive sensors. Adv. Funct. Mater. 24, 7161–7172. 10.1002/adfm.201401377 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

