CD8+ T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease
- PMID: 31709830
- PMCID: PMC7052570
- DOI: 10.1152/ajpgi.00040.2019
CD8+ T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease
Abstract
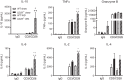
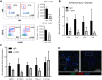
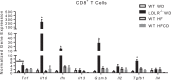
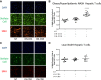
Nonalcoholic steatohepatitis (NASH) has increased in Western countries due to the prevalence of obesity. Current interests are aimed at identifying the type and function of immune cells that infiltrate the liver and key factors responsible for mediating their recruitment and activation in NASH. We investigated the function and phenotype of CD8+ T cells under obese and nonobese NASH conditions. We found an elevation in CD8 staining in livers from obese human subjects with NASH and cirrhosis that positively correlated with α-smooth muscle actin, a marker of hepatic stellate cell (HSC) activation. CD8+ T cells were elevated 3.5-fold in the livers of obese and hyperlipidemic NASH mice compared with obese hepatic steatosis mice. Isolated hepatic CD8+ T cells from these mice expressed a cytotoxic IL-10-expressing phenotype, and depletion of CD8+ T cells led to significant reductions in hepatic inflammation, HSC activation, and macrophage accumulation. Furthermore, hepatic CD8+ T cells from obese and hyperlipidemic NASH mice activated HSCs in vitro and in vivo. Interestingly, in the lean NASH mouse model, depletion and knockdown of CD8+ T cells did not impact liver inflammation or HSC activation. We demonstrated that under obese/hyperlipidemia conditions, CD8+ T cell are key regulators of the progression of NASH, while under nonobese conditions they play a minimal role in driving the disease. Thus, therapies targeting CD8+ T cells may be a novel approach for treatment of obesity-associated NASH.NEW & NOTEWORTHY Our study demonstrates that CD8+ T cells are the primary hepatic T cell population, are elevated in obese models of NASH, and directly activate hepatic stellate cells. In contrast, we find CD8+ T cells from lean NASH models do not regulate NASH-associated inflammation or stellate cell activation. Thus, for the first time to our knowledge, we demonstrate that hepatic CD8+ T cells are key players in obesity-associated NASH.
Keywords: CD8+ T cells; lymphocytes; nonalcoholic steatohepatitis; stellate cells.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
-
- Bieghs V, Van Gorp PJ, Wouters K, Hendrikx T, Gijbels MJ, van Bilsen M, Bakker J, Binder CJ, Lütjohann D, Staels B, Hofker MH, Shiri-Sverdlov R. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS One 7: e30668, 2012. doi: 10.1371/journal.pone.0030668. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

