Metabolic Remodeling Promotes Cardiac Hypertrophy by Directing Glucose to Aspartate Biosynthesis
- PMID: 31709908
- PMCID: PMC8448129
- DOI: 10.1161/CIRCRESAHA.119.315483
Metabolic Remodeling Promotes Cardiac Hypertrophy by Directing Glucose to Aspartate Biosynthesis
Abstract
Rationale: Hypertrophied hearts switch from mainly using fatty acids (FAs) to an increased reliance on glucose for energy production. It has been shown that preserving FA oxidation (FAO) prevents the pathological shift of substrate preference, preserves cardiac function and energetics, and reduces cardiomyocyte hypertrophy during cardiac stresses. However, it remains elusive whether substrate metabolism regulates cardiomyocyte hypertrophy directly or via a secondary effect of improving cardiac energetics.
Objective: The goal of this study was to determine the mechanisms of how preservation of FAO prevents the hypertrophic growth of cardiomyocytes.
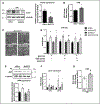
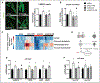
Methods and results: We cultured adult rat cardiomyocytes in a medium containing glucose and mixed-chain FAs and induced pathological hypertrophy by phenylephrine. Phenylephrine-induced hypertrophy was associated with increased glucose consumption and higher intracellular aspartate levels, resulting in increased synthesis of nucleotides, RNA, and proteins. These changes could be prevented by increasing FAO via deletion of ACC2 (acetyl-CoA-carboxylase 2) in phenylephrine-stimulated cardiomyocytes and in pressure overload-induced cardiac hypertrophy in vivo. Furthermore, aspartate supplementation was sufficient to reverse the antihypertrophic effect of ACC2 deletion demonstrating a causal role of elevated aspartate level in cardiomyocyte hypertrophy. 15N and 13C stable isotope tracing revealed that glucose but not glutamine contributed to increased biosynthesis of aspartate, which supplied nitrogen for nucleotide synthesis during cardiomyocyte hypertrophy.
Conclusions: Our data show that increased glucose consumption is required to support aspartate synthesis that drives the increase of biomass during cardiac hypertrophy. Preservation of FAO prevents the shift of metabolic flux into the anabolic pathway and maintains catabolic metabolism for energy production, thus preventing cardiac hypertrophy and improving myocardial energetics.
Keywords: aspartic acid; cardiomyocyte; glucose; hypertrophy; metabolism; nucleotides.
Figures





Comment in
-
Fueling Cardiac Hypertrophy.Circ Res. 2020 Jan 17;126(2):197-199. doi: 10.1161/CIRCRESAHA.119.316358. Epub 2020 Jan 16. Circ Res. 2020. PMID: 31944916 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

