Endoplasmic reticulum Ca2+ release causes Rieske iron-sulfur protein-mediated mitochondrial ROS generation in pulmonary artery smooth muscle cells
- PMID: 31710081
- PMCID: PMC6893167
- DOI: 10.1042/BSR20192414
Endoplasmic reticulum Ca2+ release causes Rieske iron-sulfur protein-mediated mitochondrial ROS generation in pulmonary artery smooth muscle cells
Retraction in
-
Retraction: Endoplasmic reticulum Ca2+ release causes rieske iron-sulfur protein-mediated mitochondrial ROS generation in pulmonary artery smooth muscle cells.Biosci Rep. 2020 Apr 30;40(4):BSR-20192414_RET. doi: 10.1042/BSR-20192414_RET. Biosci Rep. 2020. PMID: 32324243 Free PMC article. No abstract available.
Abstract
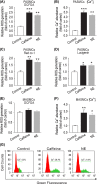
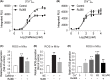
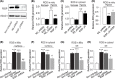
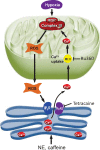
Mitochondrial reactive oxygen species (ROS) cause Ca2+ release from the endoplasmic reticulum (ER) via ryanodine receptors (RyRs) in pulmonary artery smooth muscle cells (PASMCs), playing an essential role in hypoxic pulmonary vasoconstriction (HPV). Here we tested a novel hypothesis that hypoxia-induced RyR-mediated Ca2+ release may, in turn, promote mitochondrial ROS generation contributing to hypoxic cellular responses in PASMCs. Our data reveal that application of caffeine to elevate intracellular Ca2+ concentration ([Ca2+]i) by activating RyRs results in a significant increase in ROS production in cytosol and mitochondria of PASMCs. Norepinephrine to increase [Ca2+]i due to the opening of inositol 1,4,5-triphosphate receptors (IP3Rs) produces similar effects. Exogenous Ca2+ significantly increases mitochondrial-derived ROS generation as well. Ru360 also inhibits the hypoxic ROS production. The RyR antagonist tetracaine or RyR2 gene knockout (KO) suppresses hypoxia-induced responses as well. Inhibition of mitochondrial Ca2+ uptake with Ru360 eliminates N- and Ca2+-induced responses. RISP KD abolishes the hypoxia-induced ROS production in mitochondria of PASMCs. Rieske iron-sulfur protein (RISP) gene knockdown (KD) blocks caffeine- or NE-induced ROS production. Taken together, these findings have further demonstrated that ER Ca2+ release causes mitochondrial Ca2+ uptake and RISP-mediated ROS production; this novel local ER/mitochondrion communication-elicited, Ca2+-mediated, RISP-dependent ROS production may play a significant role in hypoxic cellular responses in PASMCs.
Keywords: Intracellular calcium; Ryanodine receptor; mitochondrial ROS.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Important Role of Sarcoplasmic Reticulum Ca2+ Release via Ryanodine Receptor-2 Channel in Hypoxia-Induced Rieske Iron-Sulfur Protein-Mediated Mitochondrial Reactive Oxygen Species Generation in Pulmonary Artery Smooth Muscle Cells.Antioxid Redox Signal. 2020 Mar 1;32(7):447-462. doi: 10.1089/ars.2018.7652. Epub 2019 Oct 11. Antioxid Redox Signal. 2020. PMID: 31456413 Free PMC article.
-
Important role of PLC-γ1 in hypoxic increase in intracellular calcium in pulmonary arterial smooth muscle cells.Am J Physiol Lung Cell Mol Physiol. 2013 Feb 1;304(3):L143-51. doi: 10.1152/ajplung.00310.2012. Epub 2012 Nov 30. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23204067 Free PMC article.
-
Cross Talk Between Mitochondrial Reactive Oxygen Species and Sarcoplasmic Reticulum Calcium in Pulmonary Arterial Smooth Muscle Cells.Adv Exp Med Biol. 2017;967:289-298. doi: 10.1007/978-3-319-63245-2_17. Adv Exp Med Biol. 2017. PMID: 29047093 Review.
-
Primary role of mitochondrial Rieske iron-sulfur protein in hypoxic ROS production in pulmonary artery myocytes.Free Radic Biol Med. 2011 Apr 15;50(8):945-52. doi: 10.1016/j.freeradbiomed.2011.01.010. Epub 2011 Jan 14. Free Radic Biol Med. 2011. PMID: 21238580 Free PMC article.
-
Mitochondrial Rieske iron-sulfur protein in pulmonary artery smooth muscle: A key primary signaling molecule in pulmonary hypertension.Arch Biochem Biophys. 2019 Mar 30;664:68-75. doi: 10.1016/j.abb.2019.01.029. Epub 2019 Jan 30. Arch Biochem Biophys. 2019. PMID: 30710505 Review.
Cited by
-
Melatonin Represses Mitophagy to Protect Mouse Granulosa Cells from Oxidative Damage.Biomolecules. 2021 Jun 30;11(7):968. doi: 10.3390/biom11070968. Biomolecules. 2021. PMID: 34209255 Free PMC article.
-
Mitochondria ROS and mitophagy in acute kidney injury.Autophagy. 2023 Feb;19(2):401-414. doi: 10.1080/15548627.2022.2084862. Epub 2022 Jun 9. Autophagy. 2023. PMID: 35678504 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

