Individual differences in human opioid abuse potential as observed in a human laboratory study
- PMID: 31710994
- PMCID: PMC7219469
- DOI: 10.1016/j.drugalcdep.2019.107688
Individual differences in human opioid abuse potential as observed in a human laboratory study
Abstract
Background: Opioids have high abuse potential and pose a major public health concern. Yet, a large percentage of individuals exposed to opioids do not develop problematic use. Individual differences in opioid abuse potential are not well understood.
Methods: This within-subject (N = 16), double-blind, double-dummy, human laboratory study evaluated individual differences in response to dose (placebo, low, medium, high) following administration of heroin and hydromorphone through intravenous and subcutaneous routes, in opioid-experienced but non physically-dependent participants. Outcomes were self-reported visual analog scale (VAS) ratings (High, Liking, Drug Effect, Good Effect, Rush), pupil diameter change from baseline, and crossover point on the Drug vs. Money questionnaire. The degree to which results were consistent across measures within an individual was assessed using a mixed-effects model from which an intraclass correlation coefficient measure of between and within-subject variance was derived.
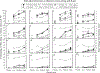
Results: The mixed effects model fit was significant (p < 0.0001) and revealed that 85.5% of the explainable variance was due to between-subject effects, suggesting the responses within an individual were highly consistent. Visual inspection reveals a myriad response pattern across participants, with some demonstrating classic dose-effect responses and others not differentiating any active doses from placebo.
Conclusions: Data suggest the abuse potential of opioids is significantly different between individuals but that the experience within an individual is highly consistent. Research to prospectively characterize and evaluate mechanisms underlying these differences is warranted and may provide a foundation to help identify persons at heightened risk of transitioning from opioid exposure to misuse and/or opioid use disorder.
Keywords: Heroin; Human individual differences; Hydromorphone; Opioid; Personalized medicine.
Copyright © 2019. Published by Elsevier B.V.
Figures
References
-
- Akaike H (1974), “A new look at the statistical model identification”, IEEE Transactions on Automatic Control, 19 (6): 716–723, doi:10.1109/TAC.1974.1100705, MR 0423716. - DOI
-
- Bates D, Maechler M, Bolker B, & Walker S 2015. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67(1), 1–48
-
- Bickel WK, Bigelow GE, Preston KL, Liebson IA, 1989. Opioid drug discrimination in humans: stability, specificity and relation to self-reported drug effect. J Pharmacol Exp Ther. 251, 1053–1063. - PubMed
-
- Bickel WK, Stitzer ML, Liebson IA, Bigelow GE, 1988. Acute physical dependence in man: effects of naloxone after brief morphine exposure. J Pharmacol Exp Ther. 244, 126–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

