The Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement: Explanation and Elaboration
- PMID: 31711094
- PMCID: PMC7750907
- DOI: 10.7326/M18-3668
The Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement: Explanation and Elaboration
Abstract
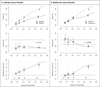
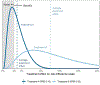
The PATH (Predictive Approaches to Treatment effect Heterogeneity) Statement was developed to promote the conduct of, and provide guidance for, predictive analyses of heterogeneity of treatment effects (HTE) in clinical trials. The goal of predictive HTE analysis is to provide patient-centered estimates of outcome risk with versus without the intervention, taking into account all relevant patient attributes simultaneously, to support more personalized clinical decision making than can be made on the basis of only an overall average treatment effect. The authors distinguished 2 categories of predictive HTE approaches (a "risk-modeling" and an "effect-modeling" approach) and developed 4 sets of guidance statements: criteria to determine when risk-modeling approaches are likely to identify clinically meaningful HTE, methodological aspects of risk-modeling methods, considerations for translation to clinical practice, and considerations and caveats in the use of effect-modeling approaches. They discuss limitations of these methods and enumerate research priorities for advancing methods designed to generate more personalized evidence. This explanation and elaboration document describes the intent and rationale of each recommendation and discusses related analytic considerations, caveats, and reservations.
Figures




References
-
- Rothwell PM. Can overall results of clinical trials be applied to all patients? Lancet 1995; 345(8965):1616–1619. - PubMed
-
- Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. Treating individuals 3: from subgroups to individuals: general principles and the example of carotid endarterectomy. Lancet 2005; 365(9455):256–265. - PubMed
-
- Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA 2007; 298(10):1209–1212. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
