Targeting Muscarinic Acetylcholine Receptors for the Treatment of Psychiatric and Neurological Disorders
- PMID: 31711626
- PMCID: PMC6941416
- DOI: 10.1016/j.tips.2019.10.007
Targeting Muscarinic Acetylcholine Receptors for the Treatment of Psychiatric and Neurological Disorders
Abstract
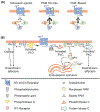
Muscarinic acetylcholine receptors (mAChR) play important roles in regulating complex behaviors such as cognition, movement, and reward, making them ideally situated as potential drug targets for the treatment of several brain disorders. Recent advances in the discovery of subtype-selective allosteric modulators for mAChRs has provided an unprecedented opportunity for highly specific modulation of signaling by individual mAChR subtypes in the brain. Recently, mAChR allosteric modulators have entered clinical development for Alzheimer's disease (AD) and schizophrenia, and have potential utility for other brain disorders. However, mAChR allosteric modulators can display a diverse array of pharmacological properties, and a more nuanced understanding of the mAChR will be necessary to best translate preclinical findings into successful clinical treatments.
Keywords: NAM; PAM; allosteric modulator; muscarinic acetylcholine receptor; neurological disorder; signal bias.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

