Clearance of Staphylococcus aureus from In Vivo Models of Chronic Infection by Immunization Requires Both Planktonic and Biofilm Antigens
- PMID: 31712267
- PMCID: PMC6921670
- DOI: 10.1128/IAI.00586-19
Clearance of Staphylococcus aureus from In Vivo Models of Chronic Infection by Immunization Requires Both Planktonic and Biofilm Antigens
Abstract
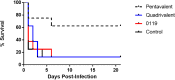
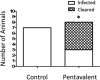
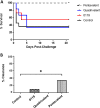
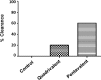
Staphylococcus aureus is a causative agent of chronic biofilm-associated infections that are recalcitrant to resolution by the immune system or antibiotics. To combat these infections, an antistaphylococcal, biofilm-specific quadrivalent vaccine against an osteomyelitis model in rabbits has previously been developed and shown to be effective at eliminating biofilm-embedded bacterial populations. However, the addition of antibiotics was required to eradicate remaining planktonic populations. In this study, a planktonic upregulated antigen was combined with the quadrivalent vaccine to remove the need for antibiotic therapy. Immunization with this pentavalent vaccine followed by intraperitoneal challenge of BALB/c mice with S. aureus resulted in 16.7% and 91.7% mortality in pentavalent vaccine and control groups, respectively (P < 0.001). Complete bacterial elimination was found in 66.7% of the pentavalent cohort, while only 8.3% of the control animals cleared the infection (P < 0.05). Further protective efficacy was observed in immunized rabbits following intramedullary challenge with S. aureus, where 62.5% of the pentavalent cohort completely cleared the infection, versus none of the control animals (P < 0.05). Passive immunization of BALB/c mice with serum IgG against the vaccine antigens prior to intraperitoneal challenge with S. aureus prevented mortality in 100% of mice and eliminated bacteria in 33.3% of the challenged mice. These results demonstrate that targeting both the planktonic and biofilm stages with the pentavalent vaccine or the IgG elicited by immunization can effectively protect against S. aureus infection.
Keywords: Staphylococcus aureus; animal model; biofilm; vaccine.
Copyright © 2019 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

