The structure of the bacterial iron-catecholate transporter Fiu suggests that it imports substrates via a two-step mechanism
- PMID: 31712312
- PMCID: PMC6926462
- DOI: 10.1074/jbc.RA119.011018
The structure of the bacterial iron-catecholate transporter Fiu suggests that it imports substrates via a two-step mechanism
Abstract
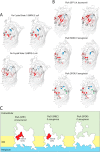
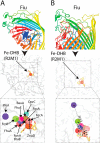
The ferric iron uptake (Fiu) transporter from Escherichia coli functions in the transport of iron-catecholate complexes across the bacterial outer membrane, providing the bacterium with iron, which is essential for growth. Recently it has become clear that Fiu also represents a liability for E. coli because its activity allows import of antimicrobial compounds that mimic catecholate. This inadvertent import suggests the potential utility of antimicrobial catechol siderophore mimetics in managing bacterial infections. However, to fully exploit these compounds, a detailed understanding of the mechanism of transport through Fiu and related transporters is required. To address this question, we determined the crystal structure of Fiu at 2.1-2.9 Å and analyzed its function in E. coli Through analysis of the Fiuo crystal structure, in combination with in silico docking and mutagenesis, we provide insight into how Fiu and related transporters bind catecholate in a surface-exposed cavity. Moreover, through determination of the structure of Fiu in multiple crystal states, we revealed the presence of a large, selectively gated cavity in the interior of this transporter. This chamber is large enough to accommodate the Fiu substrate and may allow import of substrates via a two-step mechanism. This would avoid channel formation through the transporter and inadvertent import of toxic molecules. As Fiu and its homologs are the targets of substrate-mimicking antibiotics, these results may assist in the development of these compounds.
Keywords: Escherichia coli (E. coli); TonB-dependent transporter; X-ray crystallography; bacterial outer membrane; ferric iron uptake (Fiu); iron acquisition; membrane transport; outer membrane; protein structure; siderophore; solute uptake.
© 2019 Grinter and Lithgow.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Specificity and mechanism of TonB-dependent ferric catecholate uptake by Fiu.Front Microbiol. 2024 Mar 27;15:1355253. doi: 10.3389/fmicb.2024.1355253. eCollection 2024. Front Microbiol. 2024. PMID: 38601941 Free PMC article.
-
Parasitism of iron-siderophore receptors of Escherichia coli by the siderophore-peptide microcin E492m and its unmodified counterpart.Biometals. 2006 Apr;19(2):181-91. doi: 10.1007/s10534-005-4452-9. Biometals. 2006. PMID: 16718603 Review.
-
Molecular mechanism of ferricsiderophore passage through the outer membrane receptor proteins of Escherichia coli.Biometals. 2007 Jun;20(3-4):263-74. doi: 10.1007/s10534-006-9060-9. Epub 2006 Dec 22. Biometals. 2007. PMID: 17186377 Review.
-
Structural evidence for iron-free citrate and ferric citrate binding to the TonB-dependent outer membrane transporter FecA.J Mol Biol. 2003 Sep 12;332(2):353-68. doi: 10.1016/s0022-2836(03)00855-6. J Mol Biol. 2003. PMID: 12948487
-
Iron-regulated outer membrane proteins of Escherichia coli K-12 and mechanism of action of catechol-substituted cephalosporins.Antimicrob Agents Chemother. 1988 Dec;32(12):1879-86. doi: 10.1128/AAC.32.12.1879. Antimicrob Agents Chemother. 1988. PMID: 3072926 Free PMC article.
Cited by
-
First report on the physicochemical and proteomic characterization of Proteus mirabilis outer membrane vesicles under urine-mimicking growth conditions: comparative analysis with Escherichia coli.Front Microbiol. 2024 Nov 6;15:1493859. doi: 10.3389/fmicb.2024.1493859. eCollection 2024. Front Microbiol. 2024. PMID: 39568990 Free PMC article.
-
The crystal structure of the TonB-dependent transporter YncD reveals a positively charged substrate-binding site.Acta Crystallogr D Struct Biol. 2020 May 1;76(Pt 5):484-495. doi: 10.1107/S2059798320004398. Epub 2020 Apr 27. Acta Crystallogr D Struct Biol. 2020. PMID: 32355044 Free PMC article.
-
Surveying membrane landscapes: a new look at the bacterial cell surface.Nat Rev Microbiol. 2023 Aug;21(8):502-518. doi: 10.1038/s41579-023-00862-w. Epub 2023 Feb 24. Nat Rev Microbiol. 2023. PMID: 36828896 Review.
-
Role of a TonB-dependent receptor and an oxygenase in iron-dependent copper resistance in Caulobacter crescentus.J Bacteriol. 2025 Apr 17;207(4):e0049324. doi: 10.1128/jb.00493-24. Epub 2025 Mar 14. J Bacteriol. 2025. PMID: 40084996 Free PMC article.
-
Ton motor conformational switch and peptidoglycan role in bacterial nutrient uptake.Nat Commun. 2024 Jan 6;15(1):331. doi: 10.1038/s41467-023-44606-z. Nat Commun. 2024. PMID: 38184686 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

