Template-switching mechanism of a group II intron-encoded reverse transcriptase and its implications for biological function and RNA-Seq
- PMID: 31712313
- PMCID: PMC6926447
- DOI: 10.1074/jbc.RA119.011337
Template-switching mechanism of a group II intron-encoded reverse transcriptase and its implications for biological function and RNA-Seq
Abstract
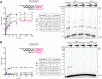
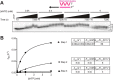
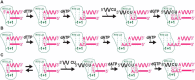
The reverse transcriptases (RTs) encoded by mobile group II introns and other non-LTR retroelements differ from retroviral RTs in being able to template-switch efficiently from the 5' end of one template to the 3' end of another with little or no complementarity between the donor and acceptor templates. Here, to establish a complete kinetic framework for the reaction and to identify conditions that more efficiently capture acceptor RNAs or DNAs, we used a
Keywords: DNA sequencing; RNA; RNA sequencing; RNA virus; RNA-dependent RNA polymerase; chemical biology; enzyme kinetics; group II intron reverse transcriptase; non-templated nucleotide addition; retrovirus; reverse transcription; structure–function; thermostable group II intron reverse transcriptase; transposable element (TE); viral polymerase.
© 2019 Lentzsch et al.
Conflict of interest statement
Thermostable group II intron reverse transcriptase enzymes and methods for their use are the subject of patents and patent applications that have been licensed by the University of Texas and East Tennessee State University to InGex, LLC. A. M. Lambowitz, some former and present members of the Lambowitz laboratory, and the University of Texas are minority equity holders in InGex, LLC, and receive royalty payments from the sale of TGIRT enzymes and kits employing TGIRT template-switching activity for RNA-seq adapter addition and from the sublicensing of intellectual property to other companies
Figures








References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

