Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma
- PMID: 31712430
- PMCID: PMC6876152
- DOI: 10.1073/pnas.1906346116
Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma
Abstract
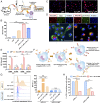
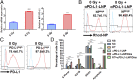
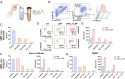
Tumor-associated myeloid cells (TAMCs) are key drivers of immunosuppression in the tumor microenvironment, which profoundly impedes the clinical response to immune-dependent and conventional therapeutic modalities. As a hallmark of glioblastoma (GBM), TAMCs are massively recruited to reach up to 50% of the brain tumor mass. Therefore, they have recently been recognized as an appealing therapeutic target to blunt immunosuppression in GBM with the hope of maximizing the clinical outcome of antitumor therapies. Here we report a nano-immunotherapy approach capable of actively targeting TAMCs in vivo. As we found that programmed death-ligand 1 (PD-L1) is highly expressed on glioma-associated TAMCs, we rationally designed a lipid nanoparticle (LNP) formulation surface-functionalized with an anti-PD-L1 therapeutic antibody (αPD-L1). We demonstrated that this system (αPD-L1-LNP) enabled effective and specific delivery of therapeutic payload to TAMCs. Specifically, encapsulation of dinaciclib, a cyclin-dependent kinase inhibitor, into PD-L1-targeted LNPs led to a robust depletion of TAMCs and an attenuation of their immunosuppressive functions. Importantly, the delivery efficiency of PD-L1-targeted LNPs was robustly enhanced in the context of radiation therapy (RT) owing to the RT-induced up-regulation of PD-L1 on glioma-infiltrating TAMCs. Accordingly, RT combined with our nano-immunotherapy led to dramatically extended survival of mice in 2 syngeneic glioma models, GL261 and CT2A. The high targeting efficiency of αPD-L1-LNP to human TAMCs from GBM patients further validated the clinical relevance. Thus, this study establishes a therapeutic approach with immense potential to improve the clinical response in the treatment of GBM and warrants a rapid translation into clinical practice.
Keywords: PD-L1; glioblastoma; immunotherapy; myeloid cell; radiotherapy.
Conflict of interest statement
Competing interest statement: There is a pending patent pertaining to the work presented in this manuscript.
Figures




References
-
- Melero I., et al. , Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 15, 457–472 (2015). - PubMed
-
- June C. H., O’Connor R. S., Kawalekar O. U., Ghassemi S., Milone M. C., CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

