mRNA structure regulates protein expression through changes in functional half-life
- PMID: 31712433
- PMCID: PMC6883848
- DOI: 10.1073/pnas.1908052116
mRNA structure regulates protein expression through changes in functional half-life
Abstract
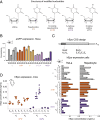
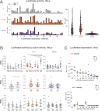
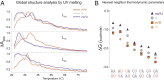
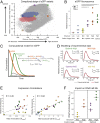
Messenger RNAs (mRNAs) encode information in both their primary sequence and their higher order structure. The independent contributions of factors like codon usage and secondary structure to regulating protein expression are difficult to establish as they are often highly correlated in endogenous sequences. Here, we used 2 approaches, global inclusion of modified nucleotides and rational sequence design of exogenously delivered constructs, to understand the role of mRNA secondary structure independent from codon usage. Unexpectedly, highly expressed mRNAs contained a highly structured coding sequence (CDS). Modified nucleotides that stabilize mRNA secondary structure enabled high expression across a wide variety of primary sequences. Using a set of eGFP mRNAs with independently altered codon usage and CDS structure, we find that the structure of the CDS regulates protein expression through changes in functional mRNA half-life (i.e., mRNA being actively translated). This work highlights an underappreciated role of mRNA secondary structure in the regulation of mRNA stability.
Keywords: RNA structure; SHAPE; mRNA therapuetics; modified nucleotides; translation.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: All authors are employees (or ex-employees in the case of S.V.S., J.R., and B.J.C.) of Moderna, Inc.
Figures

References
-
- Gustafsson C., Govindarajan S., Minshull J., Codon bias and heterologous protein expression. Trends Biotechnol. 22, 346–353 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

