Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures
- PMID: 31712445
- PMCID: PMC6883805
- DOI: 10.1073/pnas.1912129116
Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures
Abstract
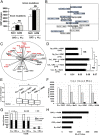
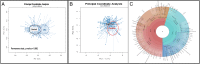
Sporadic colorectal cancer (CRC) is a result of complex interactions between the host and its environment. Environmental stressors act by causing host cell DNA alterations implicated in the onset of cancer. Here we investigate the stressor ability of CRC-associated gut dysbiosis as causal agent of host DNA alterations. The epigenetic nature of these alterations was investigated in humans and in mice. Germ-free mice receiving fecal samples from subjects with normal colonoscopy or from CRC patients were monitored for 7 or 14 wk. Aberrant crypt foci, luminal microbiota, and DNA alterations (colonic exome sequencing and methylation patterns) were monitored following human feces transfer. CRC-associated microbiota induced higher numbers of hypermethylated genes in murine colonic mucosa (vs. healthy controls' microbiota recipients). Several gene promoters including SFRP1,2,3, PENK, NPY, ALX4, SEPT9, and WIF1 promoters were found hypermethylated in CRC but not in normal tissues or effluents from fecal donors. In a pilot study (n = 266), the blood methylation levels of 3 genes (Wif1, PENK, and NPY) were shown closely associated with CRC dysbiosis. In a validation study (n = 1,000), the cumulative methylation index (CMI) of these genes was significantly higher in CRCs than in controls. Further, CMI appeared as an independent risk factor for CRC diagnosis as shown by multivariate analysis that included fecal immunochemical blood test. Consequently, fecal bacterial species in individuals with higher CMI in blood were identified by whole metagenomic analysis. Thus, CRC-related dysbiosis induces methylation of host genes, and corresponding CMIs together with associated bacteria are potential biomarkers for CRC.
Trial registration: ClinicalTrials.gov NCT01270360.
Keywords: biomarker; cancer; colon; gene methylation; microbiota.
Conflict of interest statement
Competing interest statement: I.S. shares rights in 3 patents: EP B31120, EP2635705, and EP 2955232 A1 20151216 based on methods for diagnosing adenomas and/or colorectal cancer.
Figures


References
-
- Guyton K. Z., et al. ; International Agency for Research on Cancer Monograph Working Group, IARC, Lyon, France , Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 16, 490–491 (2015). - PubMed
-
- Wild C. P., Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev. 14, 1847–1850 (2005). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

