Sirtuin 3 deficiency does not impede digit regeneration in mice
- PMID: 31712596
- PMCID: PMC6848098
- DOI: 10.1038/s41598-019-52921-z
Sirtuin 3 deficiency does not impede digit regeneration in mice
Abstract
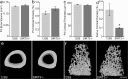
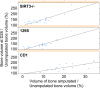
The mitochondrial deacetylase sirtuin 3 (SIRT3) is thought to be one of the main contributors to metabolic flexibility-promoting mitochondrial energy production and maintaining homeostasis. In bone, metabolic profiles are tightly regulated and the loss of SIRT3 has deleterious effects on bone volume in vivo and on osteoblast differentiation in vitro. Despite the prominent role of this protein in bone stem cell proliferation, metabolic activity, and differentiation, the importance of SIRT3 for regeneration after bone injury has never been reported. We show here, using the mouse digit amputation model, that SIRT3 deficiency has no impact on the regenerative capacity and architecture of bone and soft tissue. Regeneration occurs in SIRT3 deficient mice in spite of the reduced oxidative metabolic profile of the periosteal cells. These data suggest that bone regeneration, in contrast to homeostatic bone turnover, is not reliant upon active SIRT3, and our results highlight the need to examine known roles of SIRT3 in the context of injury.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

