Non-alcoholic fatty liver disease and cardiovascular disease: assessing the evidence for causality
- PMID: 31713012
- PMCID: PMC6946734
- DOI: 10.1007/s00125-019-05024-3
Non-alcoholic fatty liver disease and cardiovascular disease: assessing the evidence for causality
Abstract
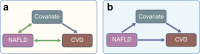
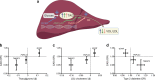
Non-alcoholic fatty liver disease (NAFLD) is highly prevalent among individuals with type 2 diabetes. Although epidemiological studies have shown that NAFLD is associated with cardiovascular disease (CVD), it remains unknown whether NAFLD is an active contributor or an innocent bystander. Plasma lipids, low-grade inflammation, impaired fibrinolysis and hepatokines are potential mediators of the relationship between NAFLD and CVD. The Mendelian randomisation approach can help to make causal inferences. Studies that used common variants in PNPLA3, TM6SF2 and GCKR as instruments to investigate the relationship between NAFLD and coronary artery disease (CAD) have reported contrasting results. Variants in PNPLA3 and TM6SF2 were found to protect against CAD, whereas variants in GCKR were positively associated with CAD. Since all three genes have been associated with non-alcoholic steatohepatitis, the second stage of NAFLD, the question of whether low-grade inflammation is an important mediator of the relationship between NAFLD and CAD arises. In contrast, the differential effects of these genes on plasma lipids (i.e. lipid-lowering for PNPLA3 and TM6SF2, and lipid-raising for GCKR) strongly suggest that plasma lipids account for their differential effects on CAD risk. This concept has recently been confirmed in an extended set of 12 NAFLD susceptibility genes. From these studies it appears that plasma lipids are an important mediator between NAFLD and CVD risk. These findings have important clinical implications, particularly for the design of anti-NAFLD drugs that also affect lipid metabolism.
Keywords: Cardiovascular disease; Coronary artery disease; GCKR; Mendelian randomisation; Non-alcoholic fatty liver disease; Non-alcoholic steatohepatitis; PNPLA3; Review; TM6SF2.
Figures

References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. - PubMed
-
- Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

