Tissue-specific Fixation Methods Are Required for Optimal In Situ Visualization of Hyaluronan in the Ovary, Kidney, and Liver
- PMID: 31714169
- PMCID: PMC6931168
- DOI: 10.1369/0022155419884879
Tissue-specific Fixation Methods Are Required for Optimal In Situ Visualization of Hyaluronan in the Ovary, Kidney, and Liver
Abstract
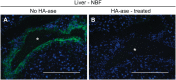
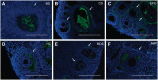
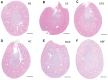
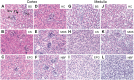
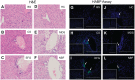
Hyaluronan (HA) is a ubiquitous component of the extracellular matrix. The spatial-temporal localization of HA can be visualized in situ using biotinylated HA binding proteins (HABPs). This assay is sensitive to fixation conditions, and there are currently no best practices for HA detection. Thus, the goal of this study was to optimize fixation conditions for visualizing HA in the ovary, kidney, and liver through analysis of six commonly used fixatives for HA detection: Bouin's Solution, Carnoy's Solution, Ethanol-Formalin-Glacial Acetic Acid (EFG), Histochoice, Modified Davidson's Solution, and 10% Neutral Buffered Formalin. Organs were harvested from CB6F1 mice and fixed with one of the identified fixatives. Fixed organs were sectioned, and the HABP assay was performed on sections in parallel. Hematoxylin and eosin staining was also performed to visualize tissue architecture. HABP signal localization and intensity varied between fixatives. EFG and Carnoy's Solution best preserved the HA signal intensity in the ovary and liver, showing HA localization in various sub-organ structures. In the kidney, only Modified Davidson's Solution was less than optimal. Our findings demonstrate that fixation can alter the ability to detect HA in tissue macro- and microstructures, as well as localization in a tissue-specific manner, in situ.
Keywords: extracellular matrix; hyaluronan binding protein assay; preservation—biological; staining/labeling.
Conflict of interest statement
Figures





Similar articles
-
Assessment of fixatives, fixation, and tissue processing on morphology and RNA integrity.Exp Mol Pathol. 2006 Apr;80(2):183-91. doi: 10.1016/j.yexmp.2005.10.002. Epub 2005 Dec 5. Exp Mol Pathol. 2006. PMID: 16332367
-
The importance of tissue fixation for light microscopic immunohistochemical localization of peroxisomal proteins: the superiority of Carnoy's fixative over Baker's formalin and Bouin's solution.Histochem Cell Biol. 1995 Mar;103(3):187-95. doi: 10.1007/BF01454023. Histochem Cell Biol. 1995. PMID: 7553132
-
Improved preservation of ovarian tissue morphology that is compatible with antigen detection using a fixative mixture of formalin and acetic acid.Hum Reprod. 2021 Jun 18;36(7):1871-1890. doi: 10.1093/humrep/deab075. Hum Reprod. 2021. PMID: 33956944 Free PMC article.
-
Viewing hyaluronan: imaging contributes to imagining new roles for this amazing matrix polymer.J Histochem Cytochem. 2011 Mar;59(3):252-7. doi: 10.1369/0022155410397760. J Histochem Cytochem. 2011. PMID: 21378279 Free PMC article. Review.
-
Densification: Hyaluronan Aggregation in Different Human Organs.Bioengineering (Basel). 2022 Apr 5;9(4):159. doi: 10.3390/bioengineering9040159. Bioengineering (Basel). 2022. PMID: 35447719 Free PMC article. Review.
Cited by
-
Temporal expression and spatial distribution of the proteoglycan versican during cardiac fibrosis development.Matrix Biol Plus. 2023 Nov 10;19-20:100135. doi: 10.1016/j.mbplus.2023.100135. eCollection 2023 Dec. Matrix Biol Plus. 2023. PMID: 38076279 Free PMC article.
-
Fixation in Form-Acetic allows hyaluronic acid detection in mouse ovaries.Reprod Fertil. 2021 Dec 10;2(4):L10-L12. doi: 10.1530/RAF-21-0085. eCollection 2021 Dec. Reprod Fertil. 2021. PMID: 35118414 Free PMC article.
-
Roles of glomerular endothelial hyaluronan in the development of proteinuria.Physiol Rep. 2021 Sep;9(17):e15019. doi: 10.14814/phy2.15019. Physiol Rep. 2021. PMID: 34472715 Free PMC article.
-
Sphingosine-1-phosphate and its mimetic FTY720 do not protect against radiation-induced ovarian fibrosis in the nonhuman primate†.Biol Reprod. 2021 May 7;104(5):1058-1070. doi: 10.1093/biolre/ioab012. Biol Reprod. 2021. PMID: 33524104 Free PMC article.
-
Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices.Aging Cell. 2020 Nov;19(11):e13259. doi: 10.1111/acel.13259. Epub 2020 Oct 20. Aging Cell. 2020. PMID: 33079460 Free PMC article.
References
-
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. - PubMed
-
- Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78. - PubMed
-
- Engström PE, Shi XQ, Tronje G, Larsson A, Welander U, Frithiof L, Engstrom GN. The effect of hyaluronan on bone and soft tissue and immune response in wound healing. J Periodontol. 2001;72:1192–200. - PubMed
-
- Cheung WF, Cruz TF, Turley EA. Receptor for hyaluronan-mediated motility (RHAMM), a hyaladherin that regulates cell responses to growth factors. Biochem Soc Trans. 1999;27:135–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

