Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson's disease models
- PMID: 31714896
- PMCID: PMC6994130
- DOI: 10.1172/JCI130767
Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson's disease models
Abstract
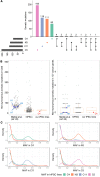
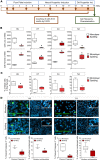
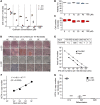
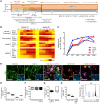
Parkinson's disease (PD) is a neurodegenerative disorder associated with loss of striatal dopamine, secondary to degeneration of midbrain dopamine (mDA) neurons in the substantia nigra, rendering cell transplantation a promising therapeutic strategy. To establish human induced pluripotent stem cell-based (hiPSC-based) autologous cell therapy, we report a platform of core techniques for the production of mDA progenitors as a safe and effective therapeutic product. First, by combining metabolism-regulating microRNAs with reprogramming factors, we developed a method to more efficiently generate clinical-grade iPSCs, as evidenced by genomic integrity and unbiased pluripotent potential. Second, we established a "spotting"-based in vitro differentiation methodology to generate functional and healthy mDA cells in a scalable manner. Third, we developed a chemical method that safely eliminates undifferentiated cells from the final product. Dopaminergic cells thus express high levels of characteristic mDA markers, produce and secrete dopamine, and exhibit electrophysiological features typical of mDA cells. Transplantation of these cells into rodent models of PD robustly restores motor function and reinnervates host brain, while showing no evidence of tumor formation or redistribution of the implanted cells. We propose that this platform is suitable for the successful implementation of human personalized autologous cell therapy for PD.
Keywords: Neuroscience; Parkinson’s disease; Stem cell transplantation; Stem cells.
Conflict of interest statement
Figures



Comment in
-
Preclinical evaluation of patient-derived cells shows promise for Parkinson's disease.J Clin Invest. 2020 Feb 3;130(2):601-603. doi: 10.1172/JCI134031. J Clin Invest. 2020. PMID: 31929191 Free PMC article.
References
-
- Kang UJ, Fahn S. Management of tardive dyskinesia. Ration Drug Ther. 1988;22(8):1–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

