Defective glycosylation and multisystem abnormalities characterize the primary immunodeficiency XMEN disease
- PMID: 31714901
- PMCID: PMC6934229
- DOI: 10.1172/JCI131116
Defective glycosylation and multisystem abnormalities characterize the primary immunodeficiency XMEN disease
Abstract
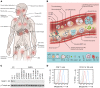
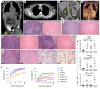
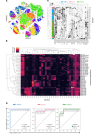
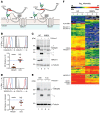
X-linked immunodeficiency with magnesium defect, EBV infection, and neoplasia (XMEN) disease are caused by deficiency of the magnesium transporter 1 (MAGT1) gene. We studied 23 patients with XMEN, 8 of whom were EBV naive. We observed lymphadenopathy (LAD), cytopenias, liver disease, cavum septum pellucidum (CSP), and increased CD4-CD8-B220-TCRαβ+ T cells (αβDNTs), in addition to the previously described features of an inverted CD4/CD8 ratio, CD4+ T lymphocytopenia, increased B cells, dysgammaglobulinemia, and decreased expression of the natural killer group 2, member D (NKG2D) receptor. EBV-associated B cell malignancies occurred frequently in EBV-infected patients. We studied patients with XMEN and patients with autoimmune lymphoproliferative syndrome (ALPS) by deep immunophenotyping (32 immune markers) using time-of-flight mass cytometry (CyTOF). Our analysis revealed that the abundance of 2 populations of naive B cells (CD20+CD27-CD22+IgM+HLA-DR+CXCR5+CXCR4++CD10+CD38+ and CD20+CD27-CD22+IgM+HLA-DR+CXCR5+CXCR4+CD10-CD38-) could differentially classify XMEN, ALPS, and healthy individuals. We also performed glycoproteomics analysis on T lymphocytes and show that XMEN disease is a congenital disorder of glycosylation that affects a restricted subset of glycoproteins. Transfection of MAGT1 mRNA enabled us to rescue proteins with defective glycosylation. Together, these data provide new clinical and pathophysiological foundations with important ramifications for the diagnosis and treatment of XMEN disease.
Keywords: Genetic diseases; Glycobiology; Immunology; Proteoglycans.
Conflict of interest statement
Figures



Comment in
-
XMEN: welcome to the glycosphere.J Clin Invest. 2020 Jan 2;130(1):80-82. doi: 10.1172/JCI134240. J Clin Invest. 2020. PMID: 31815737 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

