Identification of a novel monocytic phenotype in Classic Hodgkin Lymphoma tumor microenvironment
- PMID: 31714922
- PMCID: PMC6850552
- DOI: 10.1371/journal.pone.0224621
Identification of a novel monocytic phenotype in Classic Hodgkin Lymphoma tumor microenvironment
Abstract
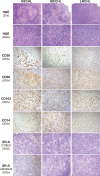
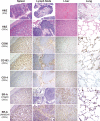
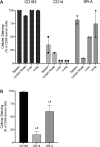
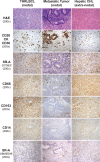
Classic Hodgkin lymphoma (CHL) characteristically shows few malignant cells in a microenvironment comprised of mixed inflammatory cells. Although CHL is associated with a high cure rate, recent studies have associated poor prognosis with absolute monocyte count in peripheral blood and increased monocyte/macrophages in involved lymph nodes. Thus, the role of monocytic infiltration and macrophage differentiation in the tumor microenvironment of CHL may be more relevant than absolute macrophage numbers to defining prognosis in CHL patients and potentially have therapeutic implications. Most studies identify tumor-associated macrophages (TAMs) using markers (e.g., CD68) expressed by macrophages and other mononuclear phagocytes, such as monocytes. In contrast, Class A Scavenger Receptor (SR-A/CD204) is expressed by tissue macrophages but not monocytic precursors. In this study, we examined SR-A expression in CHL (n = 43), and compared its expression with that of other macrophage markers. We confirmed a high prevalence of mononuclear cells that stained with CD68, CD163, and CD14 in CHL lymph nodes. However, SR-A protein expression determined by immunohistochemistry was limited to macrophages localized in sclerotic bands characteristic of nodular sclerosis CHL. In contrast, SR-A protein was readily detectable in lymph nodes with metastatic tumor, extra-nodal CHL, T cell/histiocyte-rich large B cell lymphoma, and resident macrophages in non-malignant tissues, including spleen, lymph node, liver and lung. The results of SR-A protein expression paralleled the expression of SR-A mRNA determined by quantitative RT-PCR. These data provide evidence that tumor-infiltrating monocyte/macrophages in CHL have a unique phenotype that likely depends on the microenvironment of nodal CHL.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Tumor-associated macrophages: potential role in skeletal involvement in classic Hodgkin lymphoma.J Pathol Clin Res. 2025 Jul;11(4):e70038. doi: 10.1002/2056-4538.70038. J Pathol Clin Res. 2025. PMID: 40704910 Free PMC article.
-
Tumor-associated macrophages in pediatric classical Hodgkin lymphoma: association with Epstein-Barr virus, lymphocyte subsets, and prognostic impact.Clin Cancer Res. 2012 Jul 15;18(14):3762-71. doi: 10.1158/1078-0432.CCR-12-0129. Epub 2012 May 29. Clin Cancer Res. 2012. PMID: 22645050
-
Macrophage polarization reflects T cell composition of tumor microenvironment in pediatric classical Hodgkin lymphoma and has impact on survival.PLoS One. 2015 May 15;10(5):e0124531. doi: 10.1371/journal.pone.0124531. eCollection 2015. PLoS One. 2015. PMID: 25978381 Free PMC article.
-
Role of CD68 in the tumor immune microenvironment in Hodgkin's lymphoma.Expert Rev Clin Immunol. 2024 Aug;20(8):811-819. doi: 10.1080/1744666X.2023.2294943. Epub 2023 Dec 15. Expert Rev Clin Immunol. 2024. PMID: 38087440 Review.
-
The classical Hodgkin lymphoma tumor microenvironment: macrophages and gene expression-based modeling.Hematology Am Soc Hematol Educ Program. 2014 Dec 5;2014(1):144-50. doi: 10.1182/asheducation-2014.1.144. Epub 2014 Nov 18. Hematology Am Soc Hematol Educ Program. 2014. PMID: 25696847 Review.
Cited by
-
18F-Fluorothymidine PET is an early and superior predictor of progression-free survival following chemoimmunotherapy of diffuse large B cell lymphoma: a multicenter study.Eur J Nucl Med Mol Imaging. 2021 Aug;48(9):2883-2893. doi: 10.1007/s00259-021-05353-9. Epub 2021 Apr 28. Eur J Nucl Med Mol Imaging. 2021. PMID: 33909086 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

