More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling
- PMID: 31714961
- PMCID: PMC6855112
- DOI: 10.1182/bloodadvances.2019000449
More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling
Abstract
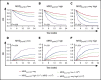
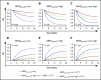
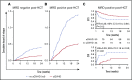
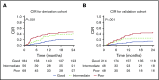
Detection of minimal residual disease (MRD) pre- and post-hematopoietic cell transplantation (HCT) for pediatric acute lymphoblastic leukemia (ALL) has been associated with relapse and poor survival. Published studies have had insufficient numbers to: (1) compare the prognostic value of pre-HCT and post-HCT MRD; (2) determine clinical factors post-HCT associated with better outcomes in MRD+ patients; and (3) use MRD and other clinical factors to develop and validate a prognostic model for relapse in pediatric patients with ALL who undergo allogeneic HCT. To address these issues, we assembled an international database including sibling (n = 191), unrelated (n = 259), mismatched (n = 56), and cord blood (n = 110) grafts given after myeloablative conditioning. Although high and very high MRD pre-HCT were significant predictors in univariate analysis, with bivariate analysis using MRD pre-HCT and post-HCT, MRD pre-HCT at any level was less predictive than even low-level MRD post-HCT. Patients with MRD pre-HCT must become MRD low/negative at 1 to 2 months and negative within 3 to 6 months after HCT for successful therapy. Factors associated with improved outcome of patients with detectable MRD post-HCT included acute graft-versus-host disease. We derived a risk score with an MRD cohort from Europe, North America, and Australia using negative predictive characteristics (late disease status, non-total body irradiation regimen, and MRD [high, very high]) defining good, intermediate, and poor risk groups with 2-year cumulative incidences of relapse of 21%, 38%, and 47%, respectively. We validated the score in a second, more contemporaneous cohort and noted 2-year cumulative incidences of relapse of 13%, 26%, and 47% (P < .001) for the defined risk groups.
Conflict of interest statement
Conflict-of-interest disclosure: P.B. reports institutional research grants from Riemser, Medac, Neovii; speakers bureau for Novartis and Amgen; and advisory boards for Amgen, Novartis, and Medac. A.B. reports consultation fees from Autolus, ElsaLys, and EusaPharma; travel reimbursement from Neovii; and company trial sponsorship from Novartis. M.R.V. reports advisory boards for Fate Therapeutics and B-MoGen, as well as stock options from both companies. M.J.B. reports research support from Becton Dickinson Biosciences, Amgen, and Bristol-Myers Squibb. C.P. reports research grants from Jazz, Riemser, Medac, and Neovii; speakers bureau for Riemser and Amgen; and advisory boards for Amgen and Novartis. S.A.G. reports research and/or clinical trial support from Novartis, Servier, and Kite; and consulting, study steering committees, or scientific advisory boards for Novartis, Adaptimmune, Eureka, TCR2, Juno, GlaxoSmithKline, Cellectis, Janssen, and Roche. M.A.P. reports institutional research grants from Adaptive and Miltenyi; speakers bureau for Novartis; advisory boards for Novartis; and educational activities for Novartis, Amgen, and Bellicum. The remaining authors declare no competing financial interests.
Figures

References
-
- van Dongen JJ, Seriu T, Panzer-Grümayer ER, et al. . Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352(9142):1731-1738. - PubMed
-
- Knechtli CJ, Goulden NJ, Hancock JP, et al. . Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92(11):4072-4079. - PubMed
-
- Bader P, Hancock J, Kreyenberg H, et al. . Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16(9):1668-1672. - PubMed

