Molecular insight of regorafenib treatment for colorectal cancer
- PMID: 31715423
- PMCID: PMC7491975
- DOI: 10.1016/j.ctrv.2019.101912
Molecular insight of regorafenib treatment for colorectal cancer
Abstract
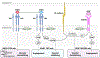
Regorafenib is a multi-targeting kinase inhibitor approved for the treatment of metastatic colorectal cancer patients in refractory to standard chemotherapy. Similarly to sorafenib, this agent was originally developed as a RAF1 inhibitor. However, the kinase inhibitory profile is distinct from sorafenib. A broad-spectrum of kinase inhibition induces wide-range drug sensitivity, irrespective of mutation status of major oncogenes. This agent's main therapeutic effects are anti-angiogenesis and the remodeling of tumor microenvironment through several mechanisms of action. The dual blockade of VEGF receptors and TIE2 can lead to both additive anti-angiogenesis effects and the suggestive unique regulation of vessel stability. Additionally, it inhibits molecular escape pathways to VEGF inhibition (e.g., FGF, PIGF, and PDGF signaling), enabling its continuous antiangiogenic effect even in tumors resistant to VEGF inhibitors. Furthermore, regorafenib has the important effect of enhancing anti-tumor immunity via macrophage modulation. Based on this concept, clinical trials have been recently launched for the development of a combination strategy with immune checkpoint inhibitors. Contrary to regorafenib induced clinical benefits and advances in the novel strategy, currently no predictive biomarkers have been identified. In the present review, we revisit and summarize regorafenib's unique mechanisms of action. The review could highlight molecular insights and provide some perspective for the search of predictive biomarkers used in metastatic colorectal cancer patients treated with regorafenib.
Keywords: Anti-angiogenesis; Biomarker; Colorectal cancer; Immunotherapy; Regorafenib; Tumor microenvironment.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest
Heinz-Josef Lenz reports receiving speakers bureau honoraria from and is a consultant/advisory board member for Merck Serono, Bayer, and Genentech. The other authors have declared no conflicts of interest.
Figures


References
-
- GLOBOCAN. All cancer fact sheet 2018, http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. 2018.
-
- Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15(10):1065–75. 10.1016/s1470-2045(14)70330-4. - DOI - PubMed
-
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129(1):245–55. 10.1002/ijc.25864. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

