Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2)
- PMID: 31715629
- PMCID: PMC7340357
- DOI: 10.1093/eurheartj/ehz772
Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2)
Abstract
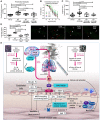
Aims: Electronic (e)-cigarettes have been marketed as a 'healthy' alternative to traditional combustible cigarettes and as an effective method of smoking cessation. There are, however, a paucity of data to support these claims. In fact, e-cigarettes are implicated in endothelial dysfunction and oxidative stress in the vasculature and the lungs. The mechanisms underlying these side effects remain unclear. Here, we investigated the effects of e-cigarette vapour on vascular function in smokers and experimental animals to determine the underlying mechanisms.
Methods and results: Acute e-cigarette smoking produced a marked impairment of endothelial function in chronic smokers determined by flow-mediated dilation. In mice, e-cigarette vapour without nicotine had more detrimental effects on endothelial function, markers of oxidative stress, inflammation, and lipid peroxidation than vapour containing nicotine. These effects of e-cigarette vapour were largely absent in mice lacking phagocytic NADPH oxidase (NOX-2) or upon treatment with the endothelin receptor blocker macitentan or the FOXO3 activator bepridil. We also established that the e-cigarette product acrolein, a reactive aldehyde, recapitulated many of the NOX-2-dependent effects of e-cigarette vapour using in vitro blood vessel incubation.
Conclusions: E-cigarette vapour exposure increases vascular, cerebral, and pulmonary oxidative stress via a NOX-2-dependent mechanism. Our study identifies the toxic aldehyde acrolein as a key mediator of the observed adverse vascular consequences. Thus, e-cigarettes have the potential to induce marked adverse cardiovascular, pulmonary, and cerebrovascular consequences. Since e-cigarette use is increasing, particularly amongst youth, our data suggest that aggressive steps are warranted to limit their health risks.
Keywords: Oxidative stress; Behavioural risk factor; E-cigarette vapour; Endothelial dysfunction; Lifestyle drug.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





Comment in
-
E-vaporating benefits of e-vaping.Eur Heart J. 2020 Jul 7;41(26):2484-2486. doi: 10.1093/eurheartj/ehz875. Eur Heart J. 2020. PMID: 31803927 Free PMC article. No abstract available.
-
Acute vs. chronic effects of e-cigarettes on vascular function.Eur Heart J. 2020 Apr 14;41(15):1525. doi: 10.1093/eurheartj/ehaa073. Eur Heart J. 2020. PMID: 32077923 No abstract available.
-
Long-term cardiovascular risk of e-cigarettes.Eur Heart J. 2020 Apr 14;41(15):1526. doi: 10.1093/eurheartj/ehaa079. Eur Heart J. 2020. PMID: 32077936 No abstract available.
-
Acrolein, e-cigarettes, and pulmonary and vascular damage.Eur Heart J. 2020 Apr 14;41(15):1524. doi: 10.1093/eurheartj/ehaa181. Eur Heart J. 2020. PMID: 32211878 No abstract available.
-
Acrolein exposure from electronic cigarettes.Eur Heart J. 2020 Apr 14;41(15):1523. doi: 10.1093/eurheartj/ehaa002. Eur Heart J. 2020. PMID: 32211887 Free PMC article. No abstract available.
-
No keto for AML stem cells!Blood. 2020 Sep 10;136(11):1219-1221. doi: 10.1182/blood.2020006733. Blood. 2020. PMID: 32957117 No abstract available.
References
-
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH.. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017;389:1907–1918. - PMC - PubMed
-
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, Goniewicz M, Wu Q, McRobbie HJ.. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019;380:629–637. - PubMed
-
- https://www.cdc.gov/mmwr/volumes/67/wr/mm6745a5.htm (27 October 2019).
-
- https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm620184.htm (27 October 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

