Dyskerin Mutations Present in Dyskeratosis Congenita Patients Increase Oxidative Stress and DNA Damage Signalling in Dictyostelium Discoideum
- PMID: 31717312
- PMCID: PMC6912284
- DOI: 10.3390/cells8111406
Dyskerin Mutations Present in Dyskeratosis Congenita Patients Increase Oxidative Stress and DNA Damage Signalling in Dictyostelium Discoideum
Abstract
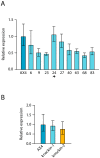
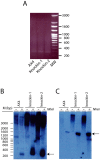
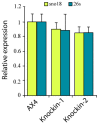
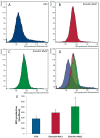
Dyskerin is a protein involved in the formation of small nucleolar and small Cajal body ribonucleoproteins. These complexes participate in RNA pseudouridylation and are also components of the telomerase complex required for telomere elongation. Dyskerin mutations cause a rare disease, X-linked dyskeratosis congenita, with no curative treatment. The social amoeba Dictyostelium discoideum contains a gene coding for a dyskerin homologous protein. In this article D. discoideum mutant strains that have mutations corresponding to mutations found in dyskeratosis congenita patients are described. The phenotype of the mutant strains has been studied and no alterations were observed in pseudouridylation activity and telomere structure. Mutant strains showed increased proliferation on liquid culture but reduced growth feeding on bacteria. The results obtained indicated the existence of increased DNA damage response and reactive oxygen species, as also reported in human Dyskeratosis congenita cells and some other disease models. These data, together with the haploid character of D. discoideum vegetative cells, that resemble the genomic structure of the human dyskerin gene, located in the X chromosome, support the conclusion that D. discoideum can be a good model system for the study of this disease.
Keywords: DNA damage; dictyostelium; dyskeratosis congenita; dyskerin; oxidative stress; pseudouridylation; telomere; telomere biology disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
GSE4, a Small Dyskerin- and GSE24.2-Related Peptide, Induces Telomerase Activity, Cell Proliferation and Reduces DNA Damage, Oxidative Stress and Cell Senescence in Dyskerin Mutant Cells.PLoS One. 2015 Nov 16;10(11):e0142980. doi: 10.1371/journal.pone.0142980. eCollection 2015. PLoS One. 2015. PMID: 26571381 Free PMC article.
-
Dyskeratosis congenita mutations in dyskerin SUMOylation consensus sites lead to impaired telomerase RNA accumulation and telomere defects.Hum Mol Genet. 2013 Sep 1;22(17):3498-507. doi: 10.1093/hmg/ddt204. Epub 2013 May 8. Hum Mol Genet. 2013. PMID: 23660516
-
Impaired Telomere Maintenance and Decreased Canonical WNT Signaling but Normal Ribosome Biogenesis in Induced Pluripotent Stem Cells from X-Linked Dyskeratosis Congenita Patients.PLoS One. 2015 May 18;10(5):e0127414. doi: 10.1371/journal.pone.0127414. eCollection 2015. PLoS One. 2015. PMID: 25992652 Free PMC article.
-
Human dyskerin: beyond telomeres.Biol Chem. 2014 Jun;395(6):593-610. doi: 10.1515/hsz-2013-0287. Biol Chem. 2014. PMID: 24468621 Review.
-
Dyskeratosis congenita: a disorder of defective telomere maintenance?Int J Hematol. 2005 Oct;82(3):184-9. doi: 10.1532/IJH97.05067. Int J Hematol. 2005. PMID: 16207588 Review.
Cited by
-
Aging, Physical Exercise, Telomeres, and Sarcopenia: A Narrative Review.Biomedicines. 2023 Feb 17;11(2):598. doi: 10.3390/biomedicines11020598. Biomedicines. 2023. PMID: 36831134 Free PMC article. Review.
-
Knock-in and precise nucleotide substitution using near-PAMless engineered Cas9 variants in Dictyostelium discoideum.Sci Rep. 2021 May 27;11(1):11163. doi: 10.1038/s41598-021-89546-0. Sci Rep. 2021. PMID: 34045481 Free PMC article.
-
Multisystemic Manifestations in Rare Diseases: The Experience of Dyskeratosis Congenita.Genes (Basel). 2022 Mar 11;13(3):496. doi: 10.3390/genes13030496. Genes (Basel). 2022. PMID: 35328050 Free PMC article. Review.
-
New Insights into Dyskerin-CypA Interaction: Implications for X-Linked Dyskeratosis Congenita and Beyond.Genes (Basel). 2023 Sep 6;14(9):1766. doi: 10.3390/genes14091766. Genes (Basel). 2023. PMID: 37761906 Free PMC article.
References
-
- Schwartz S., Bernstein D.A., Mumbach M.R., Jovanovic M., Herbst R.H., Leon-Ricardo B.X., Engreitz J.M., Guttman M., Satija R., Lander E.S., et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

