Quantitative Structure-Activity Relationship Model to Predict Antioxidant Effects of the Peptide Fraction Extracted from a Co-Culture System of Chlorella pyrenoidosa and Yarrowia lipolytica
- PMID: 31717355
- PMCID: PMC6891513
- DOI: 10.3390/md17110633
Quantitative Structure-Activity Relationship Model to Predict Antioxidant Effects of the Peptide Fraction Extracted from a Co-Culture System of Chlorella pyrenoidosa and Yarrowia lipolytica
Abstract
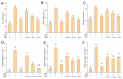
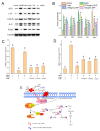
In this study, the antioxidant components in co-culture of Chlorella pyrenoidosa and Yarrowia lipolytica (3:1 ratio) were confirmed as trypsin-hydrolyzed peptides (EHPs). The EHPs were composed of 836 different peptides with molecular weights ranging from 639 to 3531 Da and were mainly composed of hydrophobic amino acids (48.1%). These peptides showed remarkable protective effects against oxidative stress in HepG2, which may be attributed to their structures. Furthermore, the mRNA and protein levels of nuclear factor erythroid 2-related factor 2 (Nrf2) were significantly lower in the peptide-treated group than in the control group, suggesting that the antioxidant enzyme-coding genes were not activated. The EC50 value of three peptides in the EHPs were in the order of AGYSPIGFVR (0.04 ± 0.002 mg/mL) > VLDELTLAR (0.09 ± 0.001 mg/mL) > LFDPVYLFDQG (0.41 ± 0.03 mg/mL); these results agreed with the prediction of the model (R2 > 0.9, Q2 > 0.5). Thus, EHPs show potential as potent new antioxidant agents.
Keywords: Chlorella pyrenoidosa; HepG2; Yarrowia lipolytica; enzymatic hydrolysis peptides; quantitative structure–activity relationship model.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
The use of serine protease from Yarrowia lipolytica yeast in the production of biopeptides from denatured egg white proteins.Acta Biochim Pol. 2017;64(2):245-253. doi: 10.18388/abp.2016_1316. Epub 2017 Apr 7. Acta Biochim Pol. 2017. PMID: 28388696
-
Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa.Biotechnol Prog. 2013 May-Jun;29(3):681-7. doi: 10.1002/btpr.1725. Epub 2013 Apr 18. Biotechnol Prog. 2013. PMID: 23606619
-
Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress.Food Chem Toxicol. 2012 Jul;50(7):2294-302. doi: 10.1016/j.fct.2012.04.022. Epub 2012 Apr 21. Food Chem Toxicol. 2012. PMID: 22542554
-
Structural and functional properties of food protein-derived antioxidant peptides.J Food Biochem. 2019 Jan;43(1):e12761. doi: 10.1111/jfbc.12761. Epub 2019 Jan 7. J Food Biochem. 2019. PMID: 31353492 Review.
-
Marine bioactive peptides as potential antioxidants.Curr Protein Pept Sci. 2013 May;14(3):189-98. doi: 10.2174/13892037113149990041. Curr Protein Pept Sci. 2013. PMID: 23721315 Review.
Cited by
-
Enhanced nitrogen removal via Yarrowia lipolytica-mediated nitrogen and related metabolism of Chlorella pyrenoidosa from wastewater.Front Bioeng Biotechnol. 2023 Jun 22;11:1159297. doi: 10.3389/fbioe.2023.1159297. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37425353 Free PMC article.
-
Effects of Collagen Hydrolysate From Large Hybrid Sturgeon on Mitigating Ultraviolet B-Induced Photodamage.Front Bioeng Biotechnol. 2022 Jun 27;10:908033. doi: 10.3389/fbioe.2022.908033. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35832410 Free PMC article.
References
-
- Cai X., Yang Q., Wang S. Antioxidant and hepatoprotective effects of pigment-protein complex from Chlorella vulgaris on carbon tetrachloride-induced liver damage in vivo. RSC Adv. 2015;116:96097–96104. doi: 10.1039/C5RA17544E. - DOI
-
- Guo Z., Tong Y.W. The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J. Appl. Phycol. 2014;26:1483–1492. doi: 10.1007/s10811-013-0186-1. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

