Anti-Osteoporotic Effects of Combined Extract of Lycii Radicis Cortex and Achyranthes japonica in Osteoblast and Osteoclast Cells and Ovariectomized Mice
- PMID: 31717518
- PMCID: PMC6893723
- DOI: 10.3390/nu11112716
Anti-Osteoporotic Effects of Combined Extract of Lycii Radicis Cortex and Achyranthes japonica in Osteoblast and Osteoclast Cells and Ovariectomized Mice
Abstract
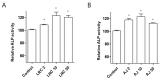
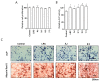
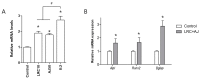
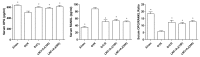
Osteoporosis is characterized by low bone density and quality with high risk of bone fracture. Here, we investigated anti-osteoporotic effects of natural plants (Lycii Radicis Cortex (LRC) and Achyranthes japonica (AJ)) in osteoblast and osteoclast cells in vitro and ovariectomized mice in vivo. Combined LRC and AJ enhanced osteoblast differentiation and mineralized bone-forming osteoblasts by the up-regulation of bone metabolic markers (Alpl, Runx2 and Bglap) in the osteoblastic cell line MC3T3-E1. However, LRC and AJ inhibited osteoclast differentiation of monocytes isolated from mouse bone marrow. In vivo experiments showed that treatment of LRC+AJ extract prevented OVX-induced trabecular bone loss and osteoclastogenesis in an osteoporotic animal model. These results suggest that LRC+AJ extract may be a good therapeutic agent for the treatment and prevention of osteoporotic bone loss.
Keywords: Achyranthes japonica; Lycii Radicis Cortex; osteoblast; osteoclast; osteoporosis; ovariectomized mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Moreira L.D., Oliveira M.L., Lirani-Galvao A.P., Marin-Mio R.V., Santos R.N., Lazaretti-Castro M. Physical exercise and osteoporosis: Effects of different types of exercises on bone and physical function of postmenopausal women. Arq. Bras. Endocrinol. Metabol. 2014;58:514–522. doi: 10.1590/0004-2730000003374. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2016K1A1A8A01939208/National Research Foundation of Korea
- 115007-03-1-SB010/High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea
- 117041-03-2-SB010/High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

