Collagen Induces a More Proliferative, Migratory and Chemoresistant Phenotype in Head and Neck Cancer via DDR1
- PMID: 31717573
- PMCID: PMC6896141
- DOI: 10.3390/cancers11111766
Collagen Induces a More Proliferative, Migratory and Chemoresistant Phenotype in Head and Neck Cancer via DDR1
Abstract
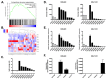
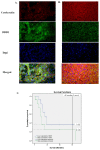
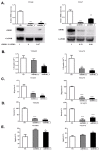
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide and includes squamous cell carcinomas of the oropharynx and oral cavity. Patient prognosis has remained poor for decades and molecular targeted therapies are not in routine use. Here we showed that the overall expression of collagen subunit genes was higher in cancer-associated fibroblasts (CAFs) than normal fibroblasts. Focusing on collagen8A1 and collagen11A1, we showed that collagen is produced by both CAFs and tumour cells, indicating that HNSCCs are collagen-rich environments. We then focused on discoidin domain receptor 1 (DDR1), a collagen-activated receptor tyrosine kinase, and showed that it is over-expressed in HNSCC tissues. Further, we demonstrated that collagen promoted the proliferation and migration of HNSCC cells and attenuated the apoptotic response to cisplatin. Knockdown of DDR1 in HNSCC cells demonstrated that these tumour-promoting effects of collagen are mediated by DDR1. Our data suggest that specific inhibitors of DDR1 might provide novel therapeutic opportunities to treat HNSCC.
Keywords: DDR1; collagen; head and neck cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Mehanna H., Beech T., Nicholson T., El-Hariry I., McConkey C., Paleri V., Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—Systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

