Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions
- PMID: 31717803
- PMCID: PMC6862138
- DOI: 10.3390/ijms20215337
Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions
Abstract
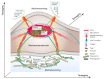
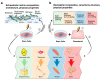
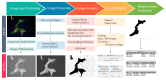
The cross-talk between stem cells and their microenvironment has been shown to have a direct impact on stem cells' decisions about proliferation, growth, migration, and differentiation. It is well known that stem cells, tissues, organs, and whole organisms change their internal architecture and composition in response to external physical stimuli, thanks to cells' ability to sense mechanical signals and elicit selected biological functions. Likewise, stem cells play an active role in governing the composition and the architecture of their microenvironment. Is now being documented that, thanks to this dynamic relationship, stemness identity and stem cell functions are maintained. In this work, we review the current knowledge in mechanobiology on stem cells. We start with the description of theoretical basis of mechanobiology, continue with the effects of mechanical cues on stem cells, development, pathology, and regenerative medicine, and emphasize the contribution in the field of the development of ex-vivo mechanobiology modelling and computational tools, which allow for evaluating the role of forces on stem cell biology.
Keywords: computational tools; ex-vivo stem cell models; mechanosensing; mechanotransduction; regenerative medicine; stem cell-biomaterial interaction; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wolff J. Das Gesetz der Transformation der Knochen. Hirschwald; Berlin, Germany: 1892.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

