Serotonin 5-HT4 Receptor Agonists Improve Facilitation of Contextual Fear Extinction in an MPTP-Induced Mouse Model of Parkinson's Disease
- PMID: 31717815
- PMCID: PMC6862438
- DOI: 10.3390/ijms20215340
Serotonin 5-HT4 Receptor Agonists Improve Facilitation of Contextual Fear Extinction in an MPTP-Induced Mouse Model of Parkinson's Disease
Abstract
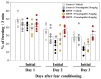
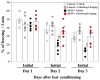
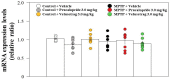
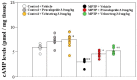
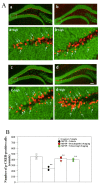
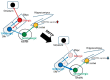
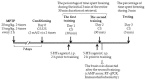
Previously, we found that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson's disease (PD) model mice (PD mice) showed facilitation of hippocampal memory extinction via reduced cyclic adenosine monophosphate (cAMP)/cAMP-dependent response element-binding protein (CREB) signaling, which may cause cognitive impairment in PD. Serotonergic neurons in the median raphe nucleus (MnRN) project to the hippocampus, and functional abnormalities have been reported. In the present study, we investigated the effects of the serotonin 5-HT4 receptor (5-HT4R) agonists prucalopride and velusetrag on the facilitation of memory extinction observed in PD mice. Both 5-HT4R agonists restored facilitation of contextual fear extinction in PD mice by stimulating the cAMP/CREB pathway in the dentate gyrus of the hippocampus. A retrograde fluorogold-tracer study showed that γ-aminobutyric acid-ergic (GABAergic) neurons in the reticular part of the substantia nigra (SNr), but not dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNpc), projected to serotonergic neurons in the MnRN, which are known to project their nerve terminals to the hippocampus. It is possible that the degeneration of the SNpc DAergic neurons in PD mice affects the SNr GABAergic neurons, and thereafter, the serotonergic neurons in the MnRN, resulting in hippocampal dysfunction. These findings suggest that 5HT4R agonists could be potentially useful as therapeutic drugs for treating cognitive deficits in PD.
Keywords: 5-HT4 receptor agonist; MPTP; Parkinson’s disease; dopamine; hippocampus; median raphe nucleus (MnRN); memory extinction; reticular part of the substantia nigra (SNr); substantia nigra pars compacta (SNpc).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hou J.G., Lai E.C. Non-motor symptoms of Parkinson’s disease. Int. J. Gerontol. 2007;1:53–64. doi: 10.1016/S1873-9598(08)70024-3. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

