Using PAR4 Inhibition as an Anti-Thrombotic Approach: Why, How, and When?
- PMID: 31717963
- PMCID: PMC6888008
- DOI: 10.3390/ijms20225629
Using PAR4 Inhibition as an Anti-Thrombotic Approach: Why, How, and When?
Abstract
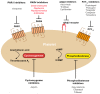
Protease-activated receptors (PARs) are a family of four GPCRs with a variety of cellular functions, yet the only advanced clinical endeavours to target these receptors for therapeutic gain to date relates to the impairment of platelet function for anti-thrombotic therapy. The only approved PAR antagonist is the PAR1 inhibitor, vorapaxar-the sole anti-platelet drug against a new target approved in the past 20 years. However, there are two PARs on human platelets, PAR1 and PAR4, and more recent efforts have focused on the development of the first PAR4 antagonists, with first-in-class agents recently beginning clinical trial. Here, we review the rationale for this approach, outline the various modes of PAR4 inhibition, and speculate on the specific therapeutic potential of targeting PAR4 for the prevention of thrombotic conditions.
Keywords: antagonists; anti-platelets; anti-thrombotics; platelet; protease-activated receptors; thrombin; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inhibition of protease-activated receptor 4 impairs platelet procoagulant activity during thrombus formation in human blood.J Thromb Haemost. 2016 Aug;14(8):1642-54. doi: 10.1111/jth.13293. Epub 2016 Jun 22. J Thromb Haemost. 2016. PMID: 26878340
-
PAR1 antagonists inhibit thrombin-induced platelet activation whilst leaving the PAR4-mediated response intact.Platelets. 2015;26(3):236-42. doi: 10.3109/09537104.2014.902924. Epub 2014 Apr 21. Platelets. 2015. PMID: 24750101
-
Approval of the first protease-activated receptor antagonist: Rationale, development, significance, and considerations of a novel anti-platelet agent.Blood Rev. 2015 May;29(3):179-89. doi: 10.1016/j.blre.2014.10.006. Epub 2014 Nov 6. Blood Rev. 2015. PMID: 25467961 Review.
-
Proteinase-activated receptors (PARs) as targets for antiplatelet therapy.Biochem Soc Trans. 2016 Apr 15;44(2):606-12. doi: 10.1042/BST20150282. Biochem Soc Trans. 2016. PMID: 27068977 Review.
-
Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis.Circulation. 2006 Mar 7;113(9):1244-54. doi: 10.1161/CIRCULATIONAHA.105.587758. Epub 2006 Feb 27. Circulation. 2006. PMID: 16505172
Cited by
-
PAR4 activation involves extracellular loop 3 and transmembrane residue Thr153.Blood. 2020 Nov 5;136(19):2217-2228. doi: 10.1182/blood.2019004634. Blood. 2020. PMID: 32575122 Free PMC article.
-
The canine activated platelet secretome (CAPS): A translational model of thrombin-evoked platelet activation response.Res Pract Thromb Haemost. 2020 Dec 3;5(1):55-68. doi: 10.1002/rth2.12450. eCollection 2021 Jan. Res Pract Thromb Haemost. 2020. PMID: 33537530 Free PMC article.
-
Genetic deletion of platelet PAR4 results in reduced thrombosis and impaired hemostatic plug stability.J Thromb Haemost. 2022 Feb;20(2):422-433. doi: 10.1111/jth.15569. Epub 2021 Nov 10. J Thromb Haemost. 2022. PMID: 34689407 Free PMC article.
-
Protease-activated receptor antagonists prevent thrombosis when dual antiplatelet therapy is insufficient in an occlusive thrombosis microfluidic model.Res Pract Thromb Haemost. 2022 Apr 11;6(3):e12703. doi: 10.1002/rth2.12703. eCollection 2022 Mar. Res Pract Thromb Haemost. 2022. PMID: 35434469 Free PMC article.
-
Platelets Contribution to Thrombin Generation in Philadelphia-Negative Myeloproliferative Neoplasms: The "Circulating Wound" Model.Int J Mol Sci. 2021 Oct 20;22(21):11343. doi: 10.3390/ijms222111343. Int J Mol Sci. 2021. PMID: 34768772 Free PMC article. Review.
References
-
- van den Merkhof L.F., Zijlstra F., Olsson H., Grip L., Veen G., Bar F.W., van den Brand M.J., Simoons M.L., Verheugt F.W. Abciximab in the treatment of acute myocardial infarction eligible for primary percutaneous transluminal coronary angioplasty. Results of the Glycoprotein Receptor Antagonist Patency Evaluation (GRAPE) pilot study. J. Am. Coll Cardiol. 1999;33:1528–1532. doi: 10.1016/S0735-1097(99)00038-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

