Asparagine Synthesis During Tobacco Leaf Curing
- PMID: 31718005
- PMCID: PMC6918383
- DOI: 10.3390/plants8110492
Asparagine Synthesis During Tobacco Leaf Curing
Abstract
Senescence is a genetically controlled mechanism that modifies leaf chemistry. This involves significant changes in the accumulation of carbon- and nitrogen-containing compounds, including asparagine through the activity of asparagine synthetases. These enzymes are required for nitrogen re-assimilation and remobilization in plants; however, their mechanisms are not fully understood. Here, we report how leaf curing-a senescence-induced process that allows tobacco leaves to dry out-modifies the asparagine metabolism. We show that leaf curing strongly alters the concentration of the four main amino acids, asparagine, glutamine, aspartate, and glutamate. We demonstrate that detached tobacco leaf or stalk curing has a different impact on the expression of asparagine synthetase genes and accumulation of asparagine. Additionally, we characterize the main asparagine synthetases involved in the production of asparagine during curing. The expression of ASN1 and ASN5 genes is upregulated during curing. The ASN1-RNAi and ASN5-RNAi tobacco plant lines display significant alterations in the accumulation of asparagine, glutamine, and aspartate relative to wild-type plants. These results support the idea that ASN1 and ASN5 are key regulators of asparagine metabolism during leaf curing.
Keywords: asparagine; asparagine synthetases; curing; nitrogen assimilation; senescence; tobacco.
Conflict of interest statement
The work subject of the publication was entirely funded by PMI and all authors are employees of Philip Morris International.
Figures

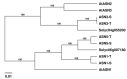




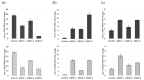
References
-
- Lothier J., Gaufichon L., Sormani R., Lemaître T., Azzopardi M., Morin H., Chardon F., Reisdorf-Cren M., Avice J.-C., Masclaux-Daubresse C. The cytosolic glutamine synthetase GLN1;2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. J. Exp. Bot. 2011;62:1375–1390. doi: 10.1093/jxb/erq299. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

