Optical Biosensors for Therapeutic Drug Monitoring
- PMID: 31718050
- PMCID: PMC6955905
- DOI: 10.3390/bios9040132
Optical Biosensors for Therapeutic Drug Monitoring
Abstract
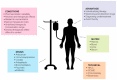
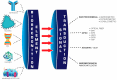
Therapeutic drug monitoring (TDM) is a fundamental tool when administering drugs that have a limited dosage or high toxicity, which could endanger the lives of patients. To carry out this monitoring, one can use different biological fluids, including blood, plasma, serum, and urine, among others. The help of specialized methodologies for TDM will allow for the pharmacodynamic and pharmacokinetic analysis of drugs and help adjust the dose before or during their administration. Techniques that are more versatile and label free for the rapid quantification of drugs employ biosensors, devices that consist of one element for biological recognition coupled to a signal transducer. Among biosensors are those of the optical biosensor type, which have been used for the quantification of different molecules of clinical interest, such as antibiotics, anticonvulsants, anti-cancer drugs, and heart failure. This review presents an overview of TDM at the global level considering various aspects and clinical applications. In addition, we review the contributions of optical biosensors to TDM.
Keywords: nanobiosensors; optical biosensors; personalized medicine; pharmacology; therapeutic drug monitoring (TDM).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Figueras A. Review of the Evidence to Include TDM in the Essential in Vitro Diagnostics List and Prioritization of Medicines to be Monitored. Fundació Institut Català de Farmacologia; Barcelona, Spain: 2019.
-
- Belmar-Liberato R., Gonzalez-Canga A., Tamame-Martin P., Escribano-Salazar M. Amoxicillin and amoxicillin-clavulanic acid resistance in veterinary medicine—the situation in Europe: A review. Vet. Med. 2011;56:473–485. doi: 10.17221/3293-VETMED. - DOI
-
- Alvarado-Miranda A., Calderillo-Ruiz G., Rodríguez-Ortiz R., Gallardo L., Aguilar-Flores K.I., Cabrera-Galeana P. Risk factors for the development of hematological toxicity during the application of weekly paclitaxel in breast cancer. Gac. Mex. Oncol. 2019;18:8–12. doi: 10.24875/j.gamo.M19000177. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

