Cardiomyocyte-Specific Snrk Prevents Inflammation in the Heart
- PMID: 31718444
- PMCID: PMC6915262
- DOI: 10.1161/JAHA.119.012792
Cardiomyocyte-Specific Snrk Prevents Inflammation in the Heart
Abstract
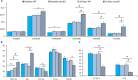
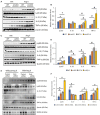
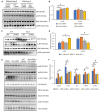
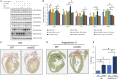
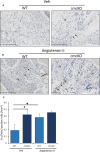
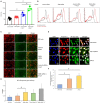
Background The SNRK (sucrose-nonfermenting-related kinase) enzyme is critical for cardiac function. However, the underlying cause for heart failure observed in Snrk cardiac conditional knockout mouse is unknown. Methods and Results Previously, 6-month adult mice knocked out for Snrk in cardiomyocytes (CMs) displayed left ventricular dysfunction. Here, 4-month adult mice, on angiotensin II (Ang II) infusion, show rapid decline in cardiac systolic function, which leads to heart failure and death in 2 weeks. These mice showed increased expression of nuclear factor κ light chain enhancer of activated B cells (NF-κB), inflammatory signaling proteins, proinflammatory proteins in the heart, and fibrosis. Interestingly, under Ang II infusion, mice knocked out for Snrk in endothelial cells did not show significant systolic or diastolic dysfunction. Although an NF-κB inflammation signaling pathway was increased in Snrk knockout endothelial cells, this did not lead to fibrosis or mortality. In hearts of adult mice knocked out for Snrk in CMs, we also observed NF-κB pathway activation in CMs, and an increased presence of Mac2+ macrophages was observed in basal and Ang II-infused states. In vitro analysis of Snrk knockdown HL-1 CMs revealed similar upregulation of the NF-κB signaling proteins and proinflammatory proteins that was exacerbated on Ang II treatment. The Ang II-induced NF-κB pathway-mediated proinflammatory effects were mediated in part through protein kinase B or AKT, wherein AKT inhibition restored the proinflammatory signaling protein levels to baseline in Snrk knockdown HL-1 CMs. Conclusions During heart failure, SNRK acts as a cardiomyocyte-specific repressor of cardiac inflammation and fibrosis.
Keywords: NF‐kB; cardiac hypertrophy; cardiomyocyte; endothelial cell; fibrosis; heart failure; inflammation.
Figures
References
-
- Giamouzis G, Kalogeropoulos A, Georgiopoulou V, Laskar S, Smith AL, Dunbar S, Triposkiadis F, Butler J. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17:54–75. - PubMed
-
- Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. - PubMed
-
- Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119:159–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

