Estimation of fibrosis progression rates for chronic hepatitis C: a systematic review and meta-analysis update
- PMID: 31719068
- PMCID: PMC6858137
- DOI: 10.1136/bmjopen-2018-027491
Estimation of fibrosis progression rates for chronic hepatitis C: a systematic review and meta-analysis update
Abstract
Objectives: Mathematical models are increasingly important in planning for the upcoming chronic hepatitis C (CHC) elimination efforts. Such models require reliable natural history inputs to make accurate predictions on health and economic outcomes. Yet, hepatitis C virus disease progression is known to vary widely in the literature and published inputs are currently outdated. The objectives of this study were to obtain updated estimates of fibrosis progression rates (FPR) in treatment-naïve patients with CHC and to explore sources of heterogeneity.
Design: A systematic review was conducted using Ovid-MEDLINE, Ovid-EMBASE and PubMed databases (January 1990 to January 2018) to identify observational studies of hepatic fibrosis in treatment-naïve patients with CHC.
Outcomes: Stage-constant FPRs were estimated for each study given the reported fibrosis scores and duration of infection. Stage-specific FPRs (ie, F0→F1; F1→F2; F2→F3; F3→F4) were estimated using Markov maximum likelihood estimation. Estimates were pooled using random-effects meta-analysis and heterogeneity was evaluated by stratification and random-effects meta-regression.
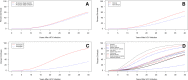
Results: The review identified 111 studies involving 131 groups of patients (n=42 693). The pooled stage-constant FPR was 0.094 (95% CI 0.088 to 0.100); stage-specific FPRs were F0→F1: 0.107 (95% CI 0.097 to 0.118); F1→F2: 0.082 (95% CI 0.074 to 0.091); F2→F3: 0.117 (95% CI 0.107 to 0.129); F3→F4: 0.116 (95% CI 0.104 to 0.131). Stratified analysis revealed substantial variation in progression by study population. Meta-regression indicated associations between progression and infection age, duration, source, viral genotype and study population. Findings indicate that FPRs display substantial heterogeneity across study populations and pooled values from more homogenous subpopulations should be considered when estimating prognosis.
Conclusions: This large meta-analysis presents updated prognostic estimates for CHC derived from newer studies using better diagnostic methods and improves estimates for important patient populations in terms of clinical policy (eg, injection drug users, non-clinical populations, liver clinic patients) and should be a valuable resource for patients, clinicians and clinical policymakers.
Keywords: cirrhosis; hepatic fibrosis; hepatitis C; viral hepatitis.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JF has received research support from AbbVie, Gilead Sciences, Janssen and Merck, Abbot and Regulus, and consulting fees from AbbVie, Gilead Sciences, Janssen and Merck.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
