Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments
- PMID: 31719150
- PMCID: PMC6937558
- DOI: 10.1074/jbc.RA119.009093
Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments
Abstract
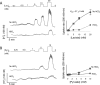
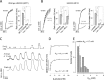
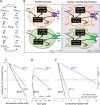
Monocarboxylate transporter 4 (MCT4) is an H+-coupled symporter highly expressed in metastatic tumors and at inflammatory sites undergoing hypoxia or the Warburg effect. At these sites, extracellular lactate contributes to malignancy and immune response evasion. Intriguingly, at 30-40 mm, the reported Km of MCT4 for lactate is more than 1 order of magnitude higher than physiological or even pathological lactate levels. MCT4 is not thought to transport pyruvate. Here we have characterized cell lactate and pyruvate dynamics using the FRET sensors Laconic and Pyronic. Dominant MCT4 permeability was demonstrated in various cell types by pharmacological means and by CRISPR/Cas9-mediated deletion. Respective Km values for lactate uptake were 1.7, 1.2, and 0.7 mm in MDA-MB-231 cells, macrophages, and HEK293 cells expressing recombinant MCT4. In MDA-MB-231 cells MCT4 exhibited a Km for pyruvate of 4.2 mm, as opposed to >150 mm reported previously. Parallel assays with the pH-sensitive dye 2',7'-bis-(carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) indicated that previous Km estimates based on substrate-induced acidification were severely biased by confounding pH-regulatory mechanisms. Numerical simulation using revised kinetic parameters revealed that MCT4, but not the related transporters MCT1 and MCT2, endows cells with the ability to export lactate in high-lactate microenvironments. In conclusion, MCT4 is a high-affinity lactate transporter with physiologically relevant affinity for pyruvate.
Keywords: Michaelis-Menten; cancer; macrophage; metabolism; monocarboxylate transporter; pyruvate; transporter.
© 2019 Contreras-Baeza et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle.J Physiol. 2000 Dec 1;529 Pt 2(Pt 2):285-93. doi: 10.1111/j.1469-7793.2000.00285.x. J Physiol. 2000. PMID: 11101640 Free PMC article.
-
The role of monocarboxylate transporter 2 and 4 in the transport of gamma-hydroxybutyric acid in mammalian cells.Drug Metab Dispos. 2007 Aug;35(8):1393-9. doi: 10.1124/dmd.107.014852. Epub 2007 May 14. Drug Metab Dispos. 2007. PMID: 17502341
-
A Holistic Evolutionary and 3D Pharmacophore Modelling Study Provides Insights into the Metabolism, Function, and Substrate Selectivity of the Human Monocarboxylate Transporter 4 (hMCT4).Int J Mol Sci. 2021 Mar 13;22(6):2918. doi: 10.3390/ijms22062918. Int J Mol Sci. 2021. PMID: 33805725 Free PMC article.
-
Current aspects of lactate exchange: lactate/H+ transport in human skeletal muscle.Eur J Appl Physiol. 2001 Nov;86(1):12-6. doi: 10.1007/s004210100517. Eur J Appl Physiol. 2001. PMID: 11820315 Review.
-
The expression of lactate transporters (MCT1 and MCT4) in heart and muscle.Eur J Appl Physiol. 2001 Nov;86(1):6-11. doi: 10.1007/s004210100516. Eur J Appl Physiol. 2001. PMID: 11820324 Review.
Cited by
-
The therapeutic importance of acid-base balance.Biochem Pharmacol. 2021 Jan;183:114278. doi: 10.1016/j.bcp.2020.114278. Epub 2020 Oct 9. Biochem Pharmacol. 2021. PMID: 33039418 Free PMC article. Review.
-
A precise prognostic signature in CTNNB1-mutant hepatocellular carcinoma: Prognosis prediction and precision treatment exploration.Heliyon. 2023 Nov 14;9(12):e22382. doi: 10.1016/j.heliyon.2023.e22382. eCollection 2023 Dec. Heliyon. 2023. PMID: 38125518 Free PMC article.
-
Role of lactate and lactate metabolism in liver diseases (Review).Int J Mol Med. 2024 Jul;54(1):59. doi: 10.3892/ijmm.2024.5383. Epub 2024 May 24. Int J Mol Med. 2024. PMID: 38785162 Free PMC article. Review.
-
Mitochondrial pyruvate carriers are required for myocardial stress adaptation.Nat Metab. 2020 Nov;2(11):1248-1264. doi: 10.1038/s42255-020-00288-1. Epub 2020 Oct 26. Nat Metab. 2020. PMID: 33106689 Free PMC article.
-
Effects of the Interaction between Rumen Microbiota Density-VFAs-Hepatic Gluconeogenesis on the Adaptability of Tibetan Sheep to Plateau.Int J Mol Sci. 2024 Jun 19;25(12):6726. doi: 10.3390/ijms25126726. Int J Mol Sci. 2024. PMID: 38928432 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

