Amino acid starvation enhances vaccine efficacy by augmenting neutralizing antibody production
- PMID: 31719173
- PMCID: PMC7271814
- DOI: 10.1126/scisignal.aav4717
Amino acid starvation enhances vaccine efficacy by augmenting neutralizing antibody production
Abstract
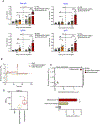
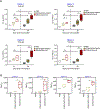
Specific reduction in the intake of proteins or amino acids (AAs) offers enormous health benefits, including increased life span, protection against age-associated disorders, and improved metabolic fitness and immunity. Cells respond to conditions of AA starvation by activating the amino acid starvation response (AAR). Here, we showed that mimicking AAR with halofuginone (HF) enhanced the magnitude and affinity of neutralizing, antigen-specific antibody responses in mice immunized with dengue virus envelope domain III protein (DENVrEDIII), a potent vaccine candidate against DENV. HF enhanced the formation of germinal centers (GCs) and increased the production of the cytokine IL-10 in the secondary lymphoid organs of vaccinated mice. Furthermore, HF promoted the transcription of genes associated with memory B cell formation and maintenance and maturation of GCs in the draining lymph nodes of vaccinated mice. The increased abundance of IL-10 in HF-preconditioned mice correlated with enhanced GC responses and may promote the establishment of long-lived plasma cells that secrete antigen-specific, high-affinity antibodies. Thus, these data suggest that mimetics of AA starvation could provide an alternative strategy to augment the efficacy of vaccines against dengue and other infectious diseases.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




References
-
- Grohmann U, Mondanelli G, Belladonna ML, Orabona C, Pallotta MT, Iacono A, Puccetti P, Volpi C, Amino-acid sensing and degrading pathways in immune regulation. Cytokine Growth Factor Rev 35, 37–45 (2017). - PubMed
-
- Saeed F, Nadeem M, Ahmed RS, Tahir Nadeem M, Arshad MS, Ullah A, Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds – a review. Food Agric. Immunol 27, 205–229 (2016).
-
- Martí A, Marcos A, a Martínez J, Obesity and immune function relationships. Obes. Rev 2, 131–140 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

