Salt-free fractionation of complex isomeric mixtures of glycosaminoglycan oligosaccharides compatible with ESI-MS and microarray analysis
- PMID: 31719635
- PMCID: PMC6851191
- DOI: 10.1038/s41598-019-53070-z
Salt-free fractionation of complex isomeric mixtures of glycosaminoglycan oligosaccharides compatible with ESI-MS and microarray analysis
Abstract
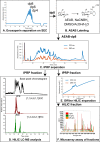
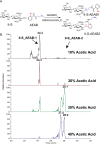
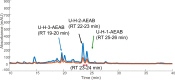
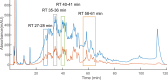
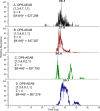
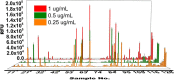
Heparin and heparan sulfate (Hp/HS) are linear complex glycosaminoglycans which are involved in diverse biological processes. The structural complexity brings difficulties in separation, making the study of structure-function relationships challenging. Here we present a separation method for Hp/HS oligosaccharide fractionation with cross-compatible solvent and conditions, combining size exclusion chromatography (SEC), ion-pair reversed phase chromatography (IPRP), and hydrophilic interaction chromatography (HILIC) as three orthogonal separation methods that do not require desalting or extensive sample handling. With this method, the final eluent is suitable for structure-function relationship studies, including tandem mass spectrometry and microarray printing. Our data indicate that high resolution is achieved on both IPRP and HILIC for Hp/HS isomers. In addition, the fractions co-eluted in IPRP could be further separated by HILIC, with both separation dimensions capable of resolving some isomeric oligosaccharides. We demonstrate this method using both unpurified reaction products from isomeric synthetic hexasaccharides and an octasaccharide fraction from enoxaparin, identifying isomers resolved by this multi-dimensional separation method. We demonstrate both structural analysis by MS, as well as functional analysis by microarray printing and screening using a prototypical Hp/HS binding protein: basic-fibroblast growth factor (FGF2). Collectively, this method provides a strategy for efficient Hp/HS structure-function characterization.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Nelson, D. L., Lehninger, A. L. & Cox, M. M. Lehninger Principles Of Biochemistry. 261–263 (Macmillan, 2012).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

