Physiological levels of the PTEN-PI3K-AKT axis activity are required for maintenance of Burkitt lymphoma
- PMID: 31719683
- PMCID: PMC7214272
- DOI: 10.1038/s41375-019-0628-0
Physiological levels of the PTEN-PI3K-AKT axis activity are required for maintenance of Burkitt lymphoma
Abstract
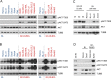
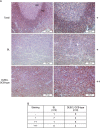
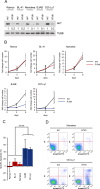
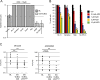
In addition to oncogenic MYC translocations, Burkitt lymphoma (BL) depends on the germinal centre (GC) dark zone (DZ) B cell survival and proliferation programme, which is characterized by relatively low PI3K-AKT activity. Paradoxically, PI3K-AKT activation facilitates MYC-driven lymphomagenesis in mice, and it has been proposed that PI3K-AKT activation is essential for BL. Here we show that the PI3K-AKT activity in primary BLs and BL cell lines does not exceed that of human non-neoplastic tonsillar GC DZ B cells. BLs were not sensitive to AKT1 knockdown, which induced massive cell death in pAKThigh DLBCL cell lines. Likewise, BL cell lines show low sensitivity to pan-AKT inhibitors. Moreover, hyper-activation of the PI3K-AKT pathway by overexpression of a constitutively active version of AKT (myrAKT) or knockdown of PTEN repressed the growth of BL cell lines. This was associated with increased AKT phosphorylation, NF-κB activation, and downregulation of DZ genes including the proto-oncogene MYB and the DZ marker CXCR4. In contrast to GCB-DLBCL, PTEN overexpression was tolerated by BL cell lines. We conclude that the molecular mechanisms instrumental to guarantee the survival of normal DZ B cells, including the tight regulation of the PTEN-PI3K-AKT axis, also operate in the survival/proliferation of BL.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Epigenetic activation of PTEN by valproic acid inhibits PI3K/AKT signaling and Burkitt lymphoma cell growth.Gene. 2025 May 20;950:149369. doi: 10.1016/j.gene.2025.149369. Epub 2025 Feb 26. Gene. 2025. PMID: 40021103
-
SPAG6 promotes cell proliferation and inhibits apoptosis through the PTEN/PI3K/AKT pathway in Burkitt lymphoma.Oncol Rep. 2020 Nov;44(5):2021-2030. doi: 10.3892/or.2020.7776. Epub 2020 Sep 22. Oncol Rep. 2020. PMID: 33000212 Free PMC article.
-
Inhibition of Hsp90 Suppresses PI3K/AKT/mTOR Signaling and Has Antitumor Activity in Burkitt Lymphoma.Mol Cancer Ther. 2017 Sep;16(9):1779-1790. doi: 10.1158/1535-7163.MCT-16-0848. Epub 2017 Jun 15. Mol Cancer Ther. 2017. PMID: 28619753 Free PMC article.
-
PI3K/PTEN/AKT Signaling Pathways in Germ Cell Development and Their Involvement in Germ Cell Tumors and Ovarian Dysfunctions.Int J Mol Sci. 2021 Sep 11;22(18):9838. doi: 10.3390/ijms22189838. Int J Mol Sci. 2021. PMID: 34575999 Free PMC article. Review.
-
PI3K/Akt signaling in urological cancers: Tumorigenesis function, therapeutic potential, and therapy response regulation.Eur J Pharmacol. 2023 Sep 15;955:175909. doi: 10.1016/j.ejphar.2023.175909. Epub 2023 Jul 23. Eur J Pharmacol. 2023. PMID: 37490949 Review.
Cited by
-
TIM-4 orchestrates mitochondrial homeostasis to promote lung cancer progression via ANXA2/PI3K/AKT/OPA1 axis.Cell Death Dis. 2023 Feb 20;14(2):141. doi: 10.1038/s41419-023-05678-3. Cell Death Dis. 2023. PMID: 36806050 Free PMC article.
-
E2F1/CKS2/PTEN signaling axis regulates malignant phenotypes in pediatric retinoblastoma.Cell Death Dis. 2022 Sep 12;13(9):784. doi: 10.1038/s41419-022-05222-9. Cell Death Dis. 2022. PMID: 36096885 Free PMC article.
-
Unraveling the complex role of neutrophils in lymphoma: From pathogenesis to therapeutic approaches (Review).Mol Clin Oncol. 2024 Sep 12;21(5):85. doi: 10.3892/mco.2024.2783. eCollection 2024 Nov. Mol Clin Oncol. 2024. PMID: 39347476 Free PMC article. Review.
-
YTHDF2 promotes temozolomide resistance in glioblastoma by activation of the Akt and NF-κB signalling pathways via inhibiting EPHB3 and TNFAIP3.Clin Transl Immunology. 2022 May 9;11(5):e1393. doi: 10.1002/cti2.1393. eCollection 2022. Clin Transl Immunology. 2022. PMID: 35582627 Free PMC article.
-
Triple targeting of RSK, AKT, and S6K as pivotal downstream effectors of PDPK1 by TAS0612 in B-cell lymphomas.Cancer Sci. 2023 Dec;114(12):4691-4705. doi: 10.1111/cas.15995. Epub 2023 Oct 15. Cancer Sci. 2023. PMID: 37840379 Free PMC article.
References
-
- Kretzmer H, Bernhart SH, Wang W, Haake A, Weniger MA, Bergmann AK, et al. DNA methylome analysis in Burkitt and follicular lymphomas identifies differentially methylated regions linked to somatic mutation and transcriptional control. Nat Genet. 2015;47:1316–25. doi: 10.1038/ng.3413. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

