Mouse Keratinocytes Without Keratin Intermediate Filaments Demonstrate Substrate Stiffness Dependent Behaviors
- PMID: 31719883
- PMCID: PMC6816603
- DOI: 10.1007/s12195-018-0526-y
Mouse Keratinocytes Without Keratin Intermediate Filaments Demonstrate Substrate Stiffness Dependent Behaviors
Abstract
Introduction: Traditionally thought to serve active vs. passive mechanical functions, respectively, a growing body of evidence suggests that actin microfilament and keratin intermediate filament (IF) networks, together with their associated cell-cell and cell-matrix anchoring junctions, may have a large degree of functional interdependence. Therefore, we hypothesized that the loss of keratin IFs in a knockout mouse keratinocyte model would affect the kinematics of colony formation, i.e., the spatiotemporal process by which individual cells join to form colonies and eventually a nascent epithelial sheet.
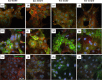
Methods: Time-lapse imaging and deformation tracking microscopy was used to observe colony formation for both wild type (WT) and keratin-deficient knockout (KO) mouse keratinocytes over 24 h. Cells were cultured under high calcium conditions on collagen-coated substrates with nominal stiffnesses of ~ 1.2 kPa (soft) and 24 kPa (stiff). Immunofluorescent staining of actin and selected adhesion proteins was also performed.
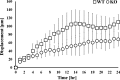
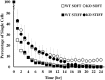
Results: The absence of keratin IFs markedly affected cell morphology, spread area, and cytoskeleton and adhesion protein organization on both soft and stiff substrates. Strikingly, an absence of keratin IFs also significantly reduced the ability of mouse keratinocytes to mechanically deform the soft substrate. Furthermore, KO cells formed colonies more efficiently on stiff vs. soft substrates, a behavior opposite to that observed for WT keratinocytes.
Conclusions: Collectively, these data are strongly supportive of the idea that an interdependence between actin microfilaments and keratin IFs does exist, while further suggesting that keratin IFs may represent an important and under-recognized component of keratinocyte mechanosensation and the force generation apparatus.
Keywords: Force; Intermediate filaments; Keratins; Mechanosensing; Polyacrylamide gels; Traction microscopy.
© Biomedical Engineering Society 2018.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

