3D Bioprinting Stem Cell Derived Tissues
- PMID: 31719887
- PMCID: PMC6816617
- DOI: 10.1007/s12195-018-0530-2
3D Bioprinting Stem Cell Derived Tissues
Abstract
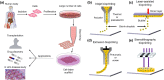
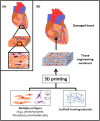
Stem cells offer tremendous promise for regenerative medicine as they can become a variety of cell types. They also continuously proliferate, providing a renewable source of cells. Recently, it has been found that 3D printing constructs using stem cells, can generate models representing healthy or diseased tissues, as well as substitutes for diseased and damaged tissues. Here, we review the current state of the field of 3D printing stem cell derived tissues. First, we cover 3D printing technologies and discuss the different types of stem cells used for tissue engineering applications. We then detail the properties required for the bioinks used when printing viable tissues from stem cells. We give relevant examples of such bioprinted tissues, including adipose tissue, blood vessels, bone, cardiac tissue, cartilage, heart valves, liver, muscle, neural tissue, and pancreas. Finally, we provide future directions for improving the current technologies, along with areas of focus for future work to translate these exciting technologies into clinical applications.
Keywords: Bioinks; Biomaterials; Controlled; Drug delivery; Pluripotent stem cells; Regenerative medicine; Stem cell niche; Tissue engineering.
© Biomedical Engineering Society 2018.
Figures




References
-
- AlGhamdi KM, Kumar A, Moussa NA. Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012;27(1):237–249. - PubMed
-
- Ali M, et al. Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication. 2014;6(4):045001. - PubMed
-
- Anil Kumar, S.A.K., S. Park, Y. Ito, B. Joddar. Photo-crosslinkable furfurl-gelatin as a novel bioink for 3D bioprinting of cardiac tissue. In: Annual Meeting of the BMES, 2017, Phoenix, Arizona, 2017.
-
- AsteriasBiotherapeutics, Asterias Biotherapeutics Announces Dosing of First Patient in New SCiSTAR Clinical Trial Cohort Testing AST-OPC1 in an Expanded Cervical Spinal Cord Injury Patient Population. 2016. Asteriasbiotherapuetics.com.
-
- Bajaj P, et al. Patterned three-dimensional encapsulation of embryonic stem cells using dielectrophoresis and stereolithography. Adv. Healthc. Mater. 2013;2(3):450–458. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

