Microparticle Depots for Controlled and Sustained Release of Endosomolytic Nanoparticles
- PMID: 31719925
- PMCID: PMC6816657
- DOI: 10.1007/s12195-019-00571-6
Microparticle Depots for Controlled and Sustained Release of Endosomolytic Nanoparticles
Abstract
Introduction: Nucleic acids have gained recognition as promising immunomodulatory therapeutics. However, their potential is limited by several drug delivery barriers, and there is a need for technologies that enhance intracellular delivery of nucleic acid drugs. Furthermore, controlled and sustained release is a significant concern, as the kinetics and localization of immunomodulators can influence resultant immune responses. Here, we describe the design and initial evaluation of poly(lactic-co-glycolic) acid (PLGA) microparticle (MP) depots for enhanced retention and sustained release of endosomolytic nanoparticles that enable the cytosolic delivery of nucleic acids.
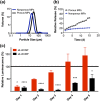
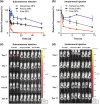
Methods: Endosomolytic p[DMAEMA]10kD-bl-[PAA0.3-co-DMAEMA0.3-co-BMA0.4]25kD diblock copolymers were synthesized by reversible addition-fragmentation chain transfer polymerization. Polymers were electrostatically complexed with nucleic acids and resultant nanoparticles (NPs) were encapsulated in PLGA MPs. To modulate release kinetics, ammonium bicarbonate was added as a porogen. Release profiles were quantified in vitro and in vivo via quantification of fluorescently-labeled nucleic acid. Bioactivity of released NPs was assessed using small interfering RNA (siRNA) targeting luciferase as a representative nucleic acid cargo. MPs were incubated with luciferase-expressing 4T1 (4T1-LUC) breast cancer cells in vitro or administered intratumorally to 4T1-LUC breast tumors, and silencing via RNA interference was quantified via longitudinal luminescence imaging.
Results: Endosomolytic NPs complexed to siRNA were effectively loaded into PLGA MPs and release kinetics could be modulated in vitro and in vivo via control of MP porosity, with porous MPs exhibiting faster cargo release. In vitro, release of NPs from porous MP depots enabled sustained luciferase knockdown in 4T1 breast cancer cells over a five-day treatment period. Administered intratumorally, MPs prolonged the retention of nucleic acid within the injected tumor, resulting in enhanced and sustained silencing of luciferase relative to a single bolus administration of NPs at an equivalent dose.
Conclusion: This work highlights the potential of PLGA MP depots as a platform for local release of endosomolytic polymer NPs that enhance the cytosolic delivery of nucleic acid therapeutics.
Keywords: Biomaterial; Drug delivery depot; Endosomal escape; Immunotherapy; Intratumoral; Local delivery; Nucleic acid therapeutics; PLGA; RNA interference.
© Biomedical Engineering Society 2019.
Figures


References
-
- Aliabadi HM. Natural polymers in nucleic acid delivery. In: Narain R, editor. Polymers and Nanomaterials for Gene Therapy. Cambridge: Woodhead Publishing; 2016. pp. 55–80.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

