Patient participation in research funding: an overview of when, why and how amongst Dutch health funds
- PMID: 31720008
- PMCID: PMC6844041
- DOI: 10.1186/s40900-019-0163-1
Patient participation in research funding: an overview of when, why and how amongst Dutch health funds
Abstract
Background: Patient participation in decision-making on health-related research has gained ground. Nineteen Dutch health-related research-funding organisations (HFs) have taken up the challenge to include patients in their funding process. A 'Patient participation (PP) advisory team' was set-up, with HF-representatives and patient advocates, who together initiated this study. We provide an overview of when, why, and how PP activities take place in HFs' funding processes, share main challenges and identify possible solutions.
Methods: A qualitative research design was used. Data was gathered by questionnaires (n = 14) and semi-structured interviews (n = 18) with HF employees responsible for patient participation, followed by a workshop (n = 27) with involved employees of HFs and key players in PP from national patient organisations and research organisations. A descriptive analysis was used for the questionnaire. A semi-directed content analysis was used for the interviews and the workshop.
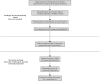
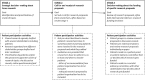
Results: Three stages can be identified in the funding process in which HFs carry out PP activities: (1) strategic decision-making about focus of research (e.g. shared research agendas); (2) call for and receipt of research proposals (e.g. mandatory inclusion of letter of recommendation from patient organisation); (3) decision-making about the funding of research proposals (e.g. patients reside in a patient panel to co-review research proposals). Main challenges identified to carry out PP activities include: how to accommodate diversity of the patient body (mainly encountered in stage 1 and 3); to what extent should patients receive training to successfully participate (mainly encountered in stage 1 and 3); and who is responsible for patient-researcher dialogues (mainly encountered in stage 1 and 2). All nineteen HFs agree that patients should be included in at least one stage of the funding process for health-related research. CONCLUSION: Further broadening and optimising patient involvement is still needed. The proposed solutions to the identified challenges could serve as inspiration for national and international research funding foundations that aim to structurally include patients in their funding process.
Keywords: Health funds; Health-related research funding; Patient inclusion; Patient involvement; Patient participation; Research funding process; Research-funding agencies; Shared decision-making.
© The Author(s). 2019.
Conflict of interest statement
Competing interestsAll authors declare that they have no competing interests.
Figures
References
-
- Teunissen GJ, Visse M a, Laan D, de Boer WI, Rutgers M, Abma T a. Patient involvement in lung foundation research: a seven year longitudinal case study. Health. 2013;5(2):320–330. doi: 10.4236/health.2013.52A043. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous