Neutrophilic Granule Protein Is a Novel Murine LPS Antagonist
- PMID: 31720045
- PMCID: PMC6829075
- DOI: 10.4110/in.2019.19.e34
Neutrophilic Granule Protein Is a Novel Murine LPS Antagonist
Abstract
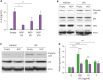
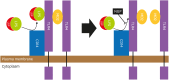
Neutrophilic granule protein (NGP) was previously reported as a granular protein of neutrophils in mouse, but the function has not been known clearly. We found the presence of the possible signal peptide in NGP and validated this protein is circulating in the bloodstream. In our findings, NGP is being modified post-translationally in Golgi apparatus and endoplasmic reticulum, which is a universal character of secretory molecules with a signal peptide. The secreted NGP protein could be detected both in vitro and in vivo. NGP has sequence similarity with an antimicrobial protein cathelicidin, and we observed the aspect of inflammation of NGP. Interestingly, NGP interacts with the complex of LPS and LPS binding protein (LBP). This interaction blocks the binding of the complex of LPS and LBP to TLR4 and the downstream inflammatory signals. Furthermore, the inhibitory function of NGP against the inflammatory effect of LPS could be observed in both in vitro and in vivo. With these findings, we report NGP is a novel secretory protein to mask LPS and inhibit its function.
Keywords: Cytokines; Inflammation; Inhibitors; Lipopolysaccharides; Neutrophil; Protein binding.
Copyright © 2019. The Korean Association of Immunologists.
Conflict of interest statement
Conflicts of Interest: The authors declare no potential conflicts of interest.
Figures
References
-
- Gazzano-Santoro H, Parent JB, Grinna L, Horwitz A, Parsons T, Theofan G, Elsbach P, Weiss J, Conlon PJ. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–4761. - PMC - PubMed
-
- Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. - PubMed
-
- Hirata M, Yoshida M, Inada K, Kirikae T. Investigation of endotoxin binding cationic proteins from granulocytes; agglutination of erythrocytes sensitized with Re-LPS. Adv Exp Med Biol. 1990;256:287–299. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

