Advances in the development and application of microbial consortia for metabolic engineering
- PMID: 31720211
- PMCID: PMC6838517
- DOI: 10.1016/j.mec.2019.e00095
Advances in the development and application of microbial consortia for metabolic engineering
Erratum in
-
Erratum regarding previously published articles in volumes 9, 10 and 11.Metab Eng Commun. 2021 Oct 28;13:e00186. doi: 10.1016/j.mec.2021.e00186. eCollection 2021 Dec. Metab Eng Commun. 2021. PMID: 34765440 Free PMC article.
Abstract
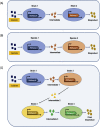
Recent advances in metabolic engineering enable the production of high-value chemicals via expressing complex biosynthetic pathways in a single microbial host. However, many engineered strains suffer from poor product yields due to redox imbalance and excess metabolic burden, and require compartmentalization of the pathway for optimal function. To address this problem, significant developments have been made towards co-cultivation of more than one engineered microbial strains to distribute metabolic burden between the co-cultivation partners and improve the product yield. In this emerging approach, metabolic pathway modules can be optimized separately in suitable hosts that will then be combined to enable optimal functionality of the complete pathway. This modular approach broadens the possibilities to fine tune sophisticated production platforms and thus achieve the biosynthesis of very complex compounds. Here, we review the different applications and the overall potential of natural and artificial co-cultivation systems in metabolic engineering in order to improve bioproduction/bioconversion. In addition to the several advantages over monocultures, major challenges and opportunities associated with co-cultivation are also discussed in this review.
Keywords: Co-cultivation; Microbial biosynthesis; Microbial consortia; Microbiome.
© 2019 The Authors.
Figures


References
-
- Benkerroum N., Daoudi A., Hamraoui T., Ghalfi H., Thiry C., Duroy M., Evrart P., Roblain D., Thonart P. Lyophilized preparations of bacteriocinogenic Lactobacillus curvatus and Lactococcus lactis subsp. lactis as potential protective adjuncts to control Listeria monocytogenes in dry-fermented sausages. J. Appl. Microbiol. 2005;98:56–63. doi: 10.1111/j.1365-2672.2004.02419.x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

