REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset
- PMID: 31720835
- PMCID: PMC6942573
- DOI: 10.1007/s00535-019-01642-1
REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset
Abstract
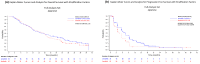
Background: A phase 3, multinational, randomized, non-inferiority trial (REFLECT) compared the efficacy and safety of lenvatinib (LEN) and sorafenib (SOR) in patients with unresectable hepatocellular carcinoma (uHCC). LEN had an effect on overall survival (OS) compared to SOR, statistically confirmed by non-inferiority [OS: median = 13.6 months vs. 12.3 months; hazard ratio (HR) 0.92, 95% confidence interval (CI) 0.79-1.06], and demonstrated statistically significant improvements in progression-free survival (PFS) and the objective response rate (ORR) in the overall population. The results of a subset analysis that evaluated the efficacy and safety of LEN and SOR in the Japanese population are reported.
Methods: The intent-to-treat population enrolled in Japan was analyzed.
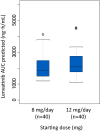
Results: Of 954 patients in the overall population, 168 Japanese patients were assigned to the LEN arm (N = 81) or the SOR arm (N = 87). Median OS was 17.6 months for LEN vs. 17.8 months for SOR (HR 0.90; 95% CI 0.62-1.29). LEN showed statistically significant improvements over SOR in PFS (7.2 months vs. 4.6 months) and ORR (29.6% vs. 6.9%). The relative dose intensity of LEN and SOR in the Japanese population was lower than in the overall population. Frequently observed, related adverse events included palmar-plantar erythrodysaesthesia syndrome (PPES), hypertension, decreased appetite, and proteinuria in the LEN arm, and PPES, hypertension, diarrhea, and alopecia in the SOR arm.
Conclusions: The efficacy and safety of LEN in the Japanese population were similar to those in the overall population of REFLECT. With manageable adverse events, LEN is a new treatment option for Japanese patients with uHCC.
Trial registration id: ClinicalTrials.gov. No. NCT01761266.
Keywords: Hepatocellular carcinoma; Japanese population; Lenvatinib; REFLECT trial; Sorafenib.
Conflict of interest statement
Tatsuya Yamashita has received honoraria from Bayer Yakuhin Ltd. and Eisai Co., Ltd. Masatoshi Kudo has held leadership positions at, or has had advisory roles at Kowa Company, Ltd., MSD, BMS, Bayer Yakuhin Ltd., Chugai Pharmaceutical Co., Ltd., and TAIHO PHARMACEUTICAL CO., LTD., has received honoraria from Bayer Yakuhin Ltd., Eisai Co., Ltd., and MSD, and has received commercial research funding from Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., TAIHO PHARMACEUTICAL CO., LTD., Sumitomo Dainippon Pharma Co., Ltd., DAIICHI SANKYO COMPANY, LIMITED., MSD, Eisai Co., Ltd., Bayer Yakuhin Ltd., and Abbvie. Kenji Ikeda has received honoraria from Dainippon Pharmaceutical Co., Ltd. and Eisai Co., Ltd. Namiki Izumi has no conflicts of interest to declare. Ryosuke Tateishi has received honoraria from Bayer Yakuhin Ltd., MSD, Eisai Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd. and has also received commercial research funding from Kyowa Hakko Kirin Co., Ltd. Masafumi Ikeda has received honoraria from Bayer Yakuhin Ltd., TAIHO PHARMACEUTICAL CO., LTD., and Novartis, and has received commercial research funding from ONO PHARMACEUTICAL CO., LTD., AstraZeneca K.K., Zeria Pharmaceutical Co., Ltd., TAIHO PHARMACEUTICAL CO., LTD., Merck Serono, Bayer Yakuhin Ltd., Yakult Honsha Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Eisai Co., Ltd., Bristol-Myers Squibb Company, Eli Lilly Japan K.K., Baxter, ASLAN Pharmaceuticals, NanoCarrier Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Hiroshi Aikata has no conflicts of interest to declare. Yasunori Kawaguchi has no conflicts of interest to declare. Yoshiyuki Wada has received honoraria from Bayer AG and commercial research funding from AstraZeneca K.K., BeiGene Ltd., Ely Lilly & Co., Pfizer Inc., and Bayer AG. Kazushi Numata has no conflicts of interest to declare. Yoshitaka Inaba has no conflicts of interest to declare. Ryoko Kuromatsu has no conflicts of interest to declare. Masahiro Kobayashi has received honoraria from Eisai Co., Ltd. Takuji Okusaka has received commercial research funding from Novartis Pharma K.K., Eli Lily Japan K.K., Dainippon Sumitomo Dainippon Pharma Co., Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and Eisai Co., Ltd. Toshiyuki Tamai is employed by Eisai Co., Ltd. Chifumi Kitamura is employed by Eisai Co., Ltd. Kenichi Saito is employed by Eisai Co., Ltd. Katsuya Haruna is employed by Eisai Co., Ltd. Kiwamu Okita has no conflicts of interest to declare. Hiromitsu Kumada has received honoraria from MSD, Sumitomo Dainippon Pharma Co., Ltd., Bristol-Myers Squibb, Abbvie, and Gilead Sciences, Inc.
Figures

References
-
- European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL–EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

