Immunogenicity of Biosimilars for Rheumatic Diseases, Plaque Psoriasis, and Inflammatory Bowel Disease: A Review from Clinical Trials and Regulatory Documents
- PMID: 31721107
- PMCID: PMC7042210
- DOI: 10.1007/s40259-019-00394-x
Immunogenicity of Biosimilars for Rheumatic Diseases, Plaque Psoriasis, and Inflammatory Bowel Disease: A Review from Clinical Trials and Regulatory Documents
Erratum in
-
Correction to: Immunogenicity of Biosimilars for Rheumatic Diseases, Plaque Psoriasis, and Inflammatory Bowel Disease: A Review from Clinical Trials and Regulatory Documents.BioDrugs. 2020 Apr;34(2):253. doi: 10.1007/s40259-020-00410-5. BioDrugs. 2020. PMID: 32103456 Free PMC article.
Abstract
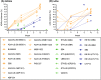
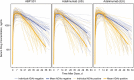
The goal of this narrative review was to summarize immunogenicity data of biosimilars or biosimilar candidates for rheumatic diseases, plaque psoriasis, or inflammatory bowel disease (IBD), available in peer-reviewed publications or regulatory documents. PubMed records and regulatory documents were searched for immunogenicity data of TNFα or CD20 inhibitor biosimilars or biosimilar candidates. Data collected included the proportion of patients positive for anti-drug antibodies (ADAbs), proportion with neutralizing antibodies (nAbs) among ADAb-positive patients, ADAb/nAb assay characteristics, cross-reactivity, and the effects of ADAbs on pharmacokinetics, pharmacodynamics, efficacy, and safety. We identified eight biosimilars or biosimilar candidates for adalimumab (BI 695501, SB5, ABP 501, GP2017, PF-06410293, MSB-11022, FKB-327, ZRC-3197) four for etanercept (SB4, GP2015, CHS-0214, LBEC0101), and three each for infliximab (SB2, CT-P13, GP1111) and rituximab (CT-P10, GP2013, PF-05280586) with immunogenicity data. Randomized, head-to-head trials with reference products varied in design and methodology of ADAb/nAb detection. The lowest proportions of ADAb-positive (0-13%) and nAb-positive patients (0-3%) were observed in the trials of etanercept and its biosimilars, and the highest with adalimumab, infliximab, and their biosimilars (ADAbs: ≤ 64%; nAbs: ≤ 100%). The most common method of ADAb detection was electrochemiluminescence, and ADAb positivity was associated with nominally inferior efficacy and safety. Overall, there were no significant immunogenicity differences between biosimilars and reference products. However, there are many discrepancies in assessing and reporting clinical immunogenicity. In conclusion, immunogenicity data of biosimilars or biosimilar candidates for TNFα or CD20 inhibitors were collected in trials that varied in design and procedures for ADAb/nAb detection. In general, immunogenicity parameters of biosimilars are similar to those of their reference products.
Conflict of interest statement
Professor Isaacs and work in the Isaacs’ laboratory are supported in part by NIHR Newcastle Biomedical Research Centre, based at Newcastle Hospitals NHS Foundation Trust and Newcastle University and by the Research into Inflammatory Arthritis Centre Versus Arthritis. V. Strand has received consulting fees or honoraria from AbbVie, Amgen, Asana, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Celltrion, EMD Serono, Genentech/Roche, GSK, Janssen, Kezar, Kypha, Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sandoz, Sanofi, and UCB. J. Gonçalves has received speaker fees from Amgen, Biogen, Celltrion, Libbs, Novartis, Pfizer, Samsung Bioepis, and Sandoz. J. D. Isaacs has received speaker or consulting fees from AbbVie, Biogen, BMS, Celltrion, Eli Lilly, Gilead, Janssen, Merck, Merck Serono, Pfizer, and Roche. T. P. Hickling is an employee of Pfizer and may own company stock. H. E. Jones and L. Marshall were employees of Pfizer during the development of the manuscript and may own company stock.
Figures

References
-
- Dörner T, Strand V, Castaneda-Hernandez G, Ferraccioli G, Isaacs JD, Kvien TK, et al. The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis. 2013;72(3):322–328. - PubMed
-
- Strand V, Kimberly R, Isaacs JD. Biologic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov. 2007;6(1):75–92. - PubMed
-
- Ellis AG, Flohr C, Drucker AM. Network meta-analyses of systemic treatments for psoriasis: a critical appraisal. Br J Dermatol. 2017;180(2):282–288. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

